-
Courses

Courses
Choosing a course is one of the most important decisions you'll ever make! View our courses and see what our students and lecturers have to say about the courses you are interested in at the links below.
-
University Life

University Life
Each year more than 4,000 choose University of Galway as their University of choice. Find out what life at University of Galway is all about here.
-
About University of Galway

About University of Galway
Since 1845, University of Galway has been sharing the highest quality teaching and research with Ireland and the world. Find out what makes our University so special – from our distinguished history to the latest news and campus developments.
-
Colleges & Schools

Colleges & Schools
University of Galway has earned international recognition as a research-led university with a commitment to top quality teaching across a range of key areas of expertise.
-
Research & Innovation

Research & Innovation
University of Galway’s vibrant research community take on some of the most pressing challenges of our times.
-
Business & Industry

Guiding Breakthrough Research at University of Galway
We explore and facilitate commercial opportunities for the research community at University of Galway, as well as facilitating industry partnership.
-
Alumni & Friends

Alumni & Friends
There are 128,000 University of Galway alumni worldwide. Stay connected to your alumni community! Join our social networks and update your details online.
-
Community Engagement

Community Engagement
At University of Galway, we believe that the best learning takes place when you apply what you learn in a real world context. That's why many of our courses include work placements or community projects.
Barry Group
Our research is focused on the development of innovative solutions for the treatment of musculoskeletal diseases, mostly degenerative diseases of joints such as osteoarthritis (OA). Our interest in OA is strongly motivated by the fact that this disease has a high prevalence and is the single most significant cause of disability worldwide. An estimated 10-15% of all adults aged over 60 have some evidence of OA, and the incidence is higher in females compared to males. It is estimated by the United Nations that 20% of the world’s population will be aged over 60 by 2050. This will inevitably lead to a greater prevalence of OA and associate disability. Worldwide, it is anticipated that 150 million people will have OA and, of these, 40 million will be severely disabled. The impact on healthcare delivery and hospital costs will continue to grow. The impact on patients and their families will also be immense since disability causes loss of income and cost burdens associated with childcare and home modifications for assisted living. In addition, there has been an alarming growth in addiction and illness associated with the long-term use of opioid medications to treat chronic pain caused by OA. Despite the severe and widespread socio-economic impact, there is a staggering lack of disease-modifying therapies. All treatments currently in use are palliative or symptom-modifying and do not address the underlying degenerative changes that occur in joints as a result of OA.
Cell Therapy for OA
The idea of using cell therapy – the transplantation of living cells – as a treatment for human disease has received considerable attention in recent years and we have been heavily involved in this effort. We have focused our attention on the use of mesenchymal stromal cells (MSCs) as a potential new medicinal product for the treatment of OA. A number of years ago, we carried out the the first study that indicated that MSCs do indeed have a regenerative effect in joint disease. Since those early studies were published, we have continued our efforts in translating this technology, with a focus on understanding the biological attributes of MSCs that make them attractive candidates for this application. We have also invested great deal of effort in understanding how MSCs can be efficiently manufactured as regulated therapeutic products, and we have been involved in clinical testing in patient trials. More recently we have turned our attention to induced pluripotent stem cells (iPSCs) as a ground-breaking development, enabling new approaches to cell-based therapies and providing unprecedented new opportunities for understanding disease mechanisms. We are currently working in the development of iPSC treatments for OA, cartilage injuries and for degenerative disc disease.
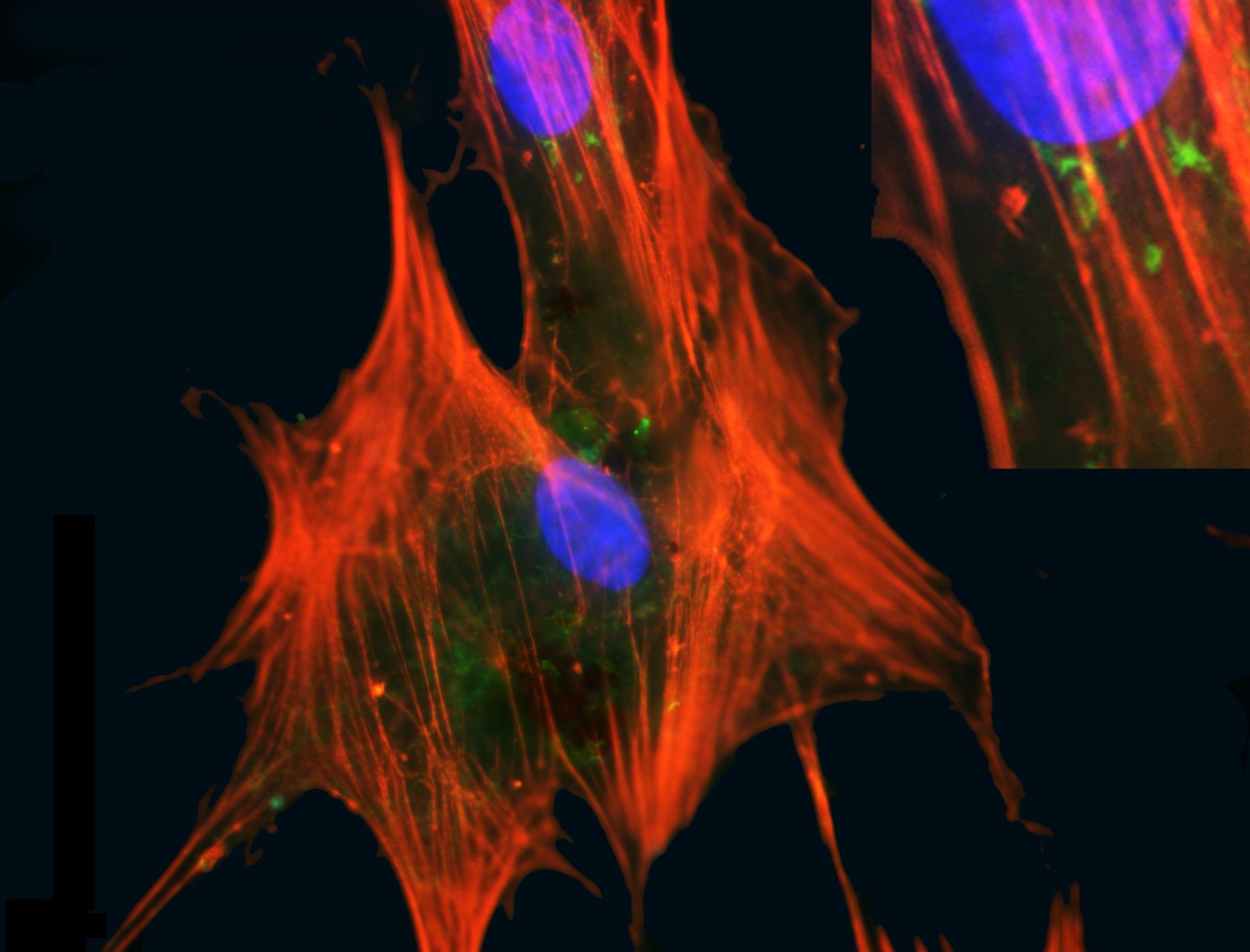
Figure 1: Mesenchymal stromal cells. MSCs are cells isolated from postnatal tissues such as bone marrow, adipose and umbilical cord that have a fibroblastic adherent morphology and can from cartilage, fat and bone tissue. They also have a capacity to modulate the immune system when delivered to a recipient. These attributes contribute to their therapeutic utility. This image shows MSCs from marrow in culture with a pronounced cytosleletal structure (orange) and nuclei (blue). Credit: Emma Mooney
Figure 2: MSC treatment for OA. MRI image of OA knee joint untreated (left) and following treatment with an intra-articular injection of MSCs. In the untreated joint there is evidence of cartilage destruction and cyst formation. In the treated joint the cartilage structure is preserved and there is no evidence of subchondral cysts. Credit: Dara Kraitchman
Figure 3: Therapeutic mechanism. Understanding the therapeutic mechanism is key to the development of MSC therapies. This image captures our current understanding of how MSCs may exert a regenerative effect in the treatment of OA and other conditions. Credit: Frank Barry.
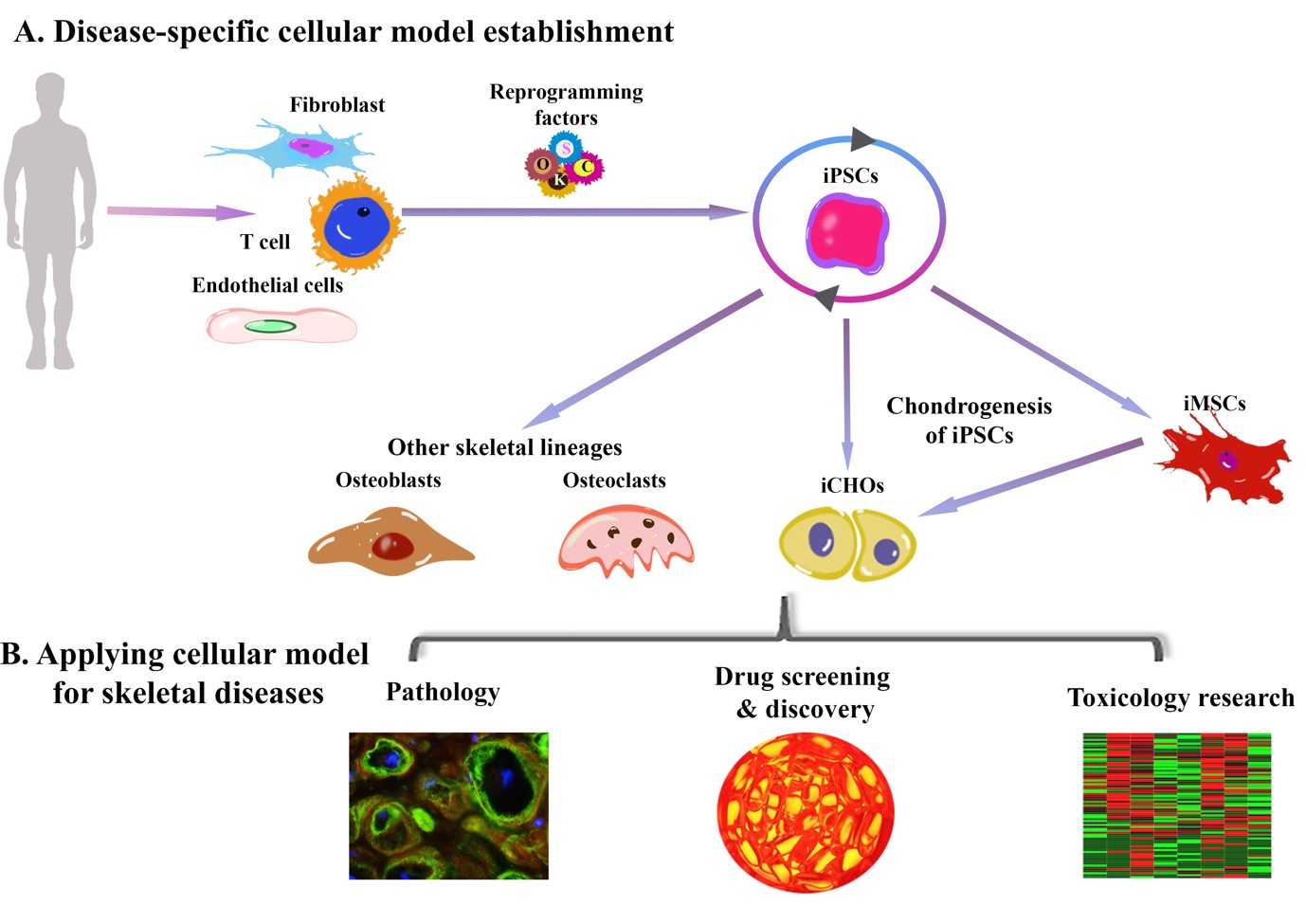
Figure 4: Schematic showing the use of iPSCs for modelling musculoskeletal disease. Credit: Aisling O’Brien
Figure 5: Clinical testing of MSC therapy for OA. This involves the procurement of a source tissue, in this case adipose tissue retrieved by liposuction (left), followed by a manufacturing process carried out under strict aseptic conditions and in compliance with current good manufacturing practice (centre), and finally delivery of the cell product to the OA joint by intra-articular injection. Credit: Frank Barry
Please see here for research publications.
Funders
- Science Foundation Ireland (SFI)
- EU Horizon 2020
- EU RDF
- Disruptive Technologies Innovation Fund (DTIF)
- Industry Awards
The Veterinary Regenerative Network
Recent advancements in veterinary medicine include the use of cell therapies as a valid therapeutic option for multiple degenerative conditions such as osteoarthritis affecting both human and veterinary patients. Based on the "One Health - One Medicine" initiative, we aim to translate our expertise with human cells for the development of high-quality and well standardized animal cell therapies.
We actively collaborate with equine and canine veterinary surgeons as part of the CALIN Veterinary Network. They give valuable insights on future clinical directions and supply the group with tissue samples such as cord blood and adipose tissue for further mesenchymal stem/stromal cell isolation and characterisation.