-
Courses

Courses
Choosing a course is one of the most important decisions you'll ever make! View our courses and see what our students and lecturers have to say about the courses you are interested in at the links below.
-
University Life

University Life
Each year more than 4,000 choose University of Galway as their University of choice. Find out what life at University of Galway is all about here.
-
About University of Galway

About University of Galway
Since 1845, University of Galway has been sharing the highest quality teaching and research with Ireland and the world. Find out what makes our University so special – from our distinguished history to the latest news and campus developments.
-
Colleges & Schools

Colleges & Schools
University of Galway has earned international recognition as a research-led university with a commitment to top quality teaching across a range of key areas of expertise.
-
Research & Innovation

Research & Innovation
University of Galway’s vibrant research community take on some of the most pressing challenges of our times.
-
Business & Industry

Guiding Breakthrough Research at University of Galway
We explore and facilitate commercial opportunities for the research community at University of Galway, as well as facilitating industry partnership.
-
Alumni & Friends

Alumni & Friends
There are 128,000 University of Galway alumni worldwide. Stay connected to your alumni community! Join our social networks and update your details online.
-
Community Engagement

Community Engagement
At University of Galway, we believe that the best learning takes place when you apply what you learn in a real world context. That's why many of our courses include work placements or community projects.
Goljanek-Whysall Group
fit-miRs as potential therapeutics for muscle wasting during ageing and disease
Researchers involved: Anthony Sannicandro, Maria Borja Gonzalez
Irreversible muscle loss and weakness are a growing issue of our ageing population with substantial socio-economic burden on European health services that will increase without the development of effective therapies. There are currently no treatments to target muscle loss despite the significant health and social issues associated with it.
Sarcopenia (age-related muscle loss), amyotrophic lateral sclerosis (a progressive disease associated with motor neuron degeneration and muscle loss) and cachexia (a progressive wasting syndrome) are multifactorial conditions, sharing several common mechanisms associated with muscle wasting, hospitalisation and decreased lifespan. microRNAs (miRNAs), potent regulators of gene expression, provide an attractive alternative strategy against muscle loss due to their simultaneous regulation of multiple gene expression without safety issues.
Using state-of-the-art omics approaches and discovery-driven bioinformatic tools, we will establish a set of conserved key miRNAs, fit-miRs, commonly dysregulated in muscle in ageing and disease. Functional studies in complementary models of in vitro and in vivo muscle loss will validate the crucial role of fit-miRs in muscle homeostasis and their potential to improve muscle mass and strength in ageing and disease. This project is funded by Irish Research Council.
Modified microRNAs as therapeutic targets for muscle wasting in sarcopenia and cachexia
Researchers involved: Raul Gonzlaez Ojeda, Rossella Giannoccari, Turki Aljuaid
Loss of skeletal muscle mass and strength with age is one of the primary underlying causes of hospitalisation and bed rest. Currently there is no effective treatment although resistance exercise has proved to delay its onset. Understanding the underlying cellular changes is essential for designing an effective therapy. Key regulatory molecules that control the level of expression of proteins within the muscle are called microRNAs. However, these molecules can be targeted by other molecules generated within the cell. Modification of microRNAs can result in a loss of specificity for their targets and pathogenesis. A vicious cycle occurs during ageing with a dysregulation of miRNAs, resulting in aberrant protein regulation. We are identifying key modified miRNAs and their protein targets, the outcomes will provide a basis for design of novel interventions against age- and cachexia-related loss of muscle. This project is in collaboration with Dr. Brian McDonagh, NUI Galway, Dr. Simon Moxon, UEA, UK and Prof. Claire Stewart, JMU, UK and Prof. Pouhlsen, Denmark. This research is funded by Science Foundation Ireland.
Improving long-term patient recovery and reducing disability after COVID-19 critical illness using microRNA-based approaches.
Researchers involved: Sarah Fagan
Ageing is the biggest risk factor for poor outcomes due to COVID-19 with approximately 58% cases hospitalised in Ireland being over 65 and many requiring ICU. Ventilator-induced diaphragm dysfunction (VIDD) and skeletal muscle wasting are found in critically ill patients within 24h of initiation of mechanical ventilation and can last over 12 months following discharge leading to frailty, loss of independence, further hospitalisation and increased morbidity and mortality. Elevated cytokine levels and immunosenescence are common in older people. COVID-19 patients with more severe ARDS show increased levels of IL6 and macrophage activation syndrome and are often administered drugs inducing muscle relaxation during mechanical ventilation leading to increased risk of muscle wasting in survivors. There is no cure for respiratory or limb muscle wasting. Irreversible muscle loss leading to frailty is a socio-economic and healthcare burden that will increase with our ageing population and is likely to become a public health priority in the light of the current pandemic. This project addresses a medical priority of treating ICU-related diaphragm and muscle weakness and establishing the potential of microRNAs as therapeutics and biomarkers of muscle loss and frailty in COVID-19 survivors to personalise and improve patient recovery. This project is in collaboration with Dr. Brian McDonagh, Prof. John Laffey, Dr. Bairbre McNicholas, Kevin O’Connell, NUI Galway/UHG, Prof. Ken O’Halloran, Dr. David Burns, UCC and Dr. Ronan O’Caoimh, Mercy University Hospital, Cork. This research is funded by Health Research Board.
microRNAs role in Osteoarthritis
Researchers involved: Rossella Giannoccari
Musculoskeletal system disorders are among the five most prevalent conditions affecting older people. Osteoarthritis (OA) is a subset of joint disorders with various aetiologies (eg. mechanical, genetic) sharing common pathophysiological processes resulting in degeneration of synovial joints. This leads to pain, disability and loss of independence. Similarly, muscle wasting directly affects the stability of the joints and loss of mobility leads to gradual degeneration of articular cartilage (AC).
Progressive loss of muscle has consequences on joint stability and health. Muscle wasting is associated with OA. Over the past few years, the relationship between cartilage and the surrounding skeletal muscle has become more evident. Already during embryogenesis, muscle contractility is required for joint formation and during OA, muscle weakness has been shown to be an important determinant of pain and disability. Several studies have shown that a decrease in lower limb lean mass is frequent in OA patients and this is associated with greater risk of falls. Moreover, it has been shown that muscle damage was associated with articular cartilage degeneration, whereas increased cross sectional area of vastus medialis was associated with improved structural changes at the knee and reduced pain in OA patients.
We propose that muscle wasting is an important component of OA pathology and by improving muscle mass and function the symptoms of OA can be ameliorated. This project is a collaboration with Prof. G. Bou-gharios, Dr. B. Poulet, Dr. M. Peffers and Prof. P. Clegg, University of Liverpool, UK
The effects of maternal diet on neuromuscular homeostasis in offspring
This project is a collaboration with Dr. Aphrodite Vasilaki and Prof. Susan Ozanne, UK.
The reduction in muscle mass and strength that occur during ageing can have a major impact on the quality of life of older individuals. Older people demonstrate loss of confidence in walking and reduced mobility which in turn leads to loss of independence and social isolation. These changes occur partly because we lose a large proportion of the muscle cells (called muscle fibres), but also the muscle cells that we retain are weak. It is currently unknown how muscle fibres are lost during ageing. There is considerable evidence that poor maternal nutrition leads to a number of changes in muscle of the offspring that result in reduced function. Muscle strength is also compromised in older individuals who did not grow well in early life, and studies suggest that maternal, developmental and nutritional factors are important. microRNAs are small molecules that regulate gene expression resulting in different sets of proteins being present in the cells. Through this, microRNAs regulate cells function. We have shown that the levels of different microRNAs change in the muscle as an effect of reduced protein diet. We propose that a reduction in protein intake during fetal and early neonatal life results in modified miRNA-target interactions in muscles of the offspring and this leads to loss of muscle mass and function which has long term effects on the number of muscle fibres and this ultimately adversely influences whether older individuals can maintain good muscle function as they age. This project is funded by BBSRC.
microRNAs role in tendon repair
Tendons connect muscles to bones and are important in how we move. Tendons frequently become injured and diseased and currently there are few, if any, treatments which have been shown to be beneficial. Tendon injuries frequently lead to painful and long-term disability and can affect the foot, the knee, the hip and the shoulder. In recent years, a new way in which cells can alter their behaviour and potentially initiate disease has been discovered. We are investigating this in particular cells in tendons, which we think may be responsible for the development of tendon injuries, to see if we can manipulate this response and prevent the injuries from developing. This project is in collaboration with Prof. P. Clegg and Dr. M. Peffers, University of Liverpool, UK and is funded by Orthopaedic Research UK.
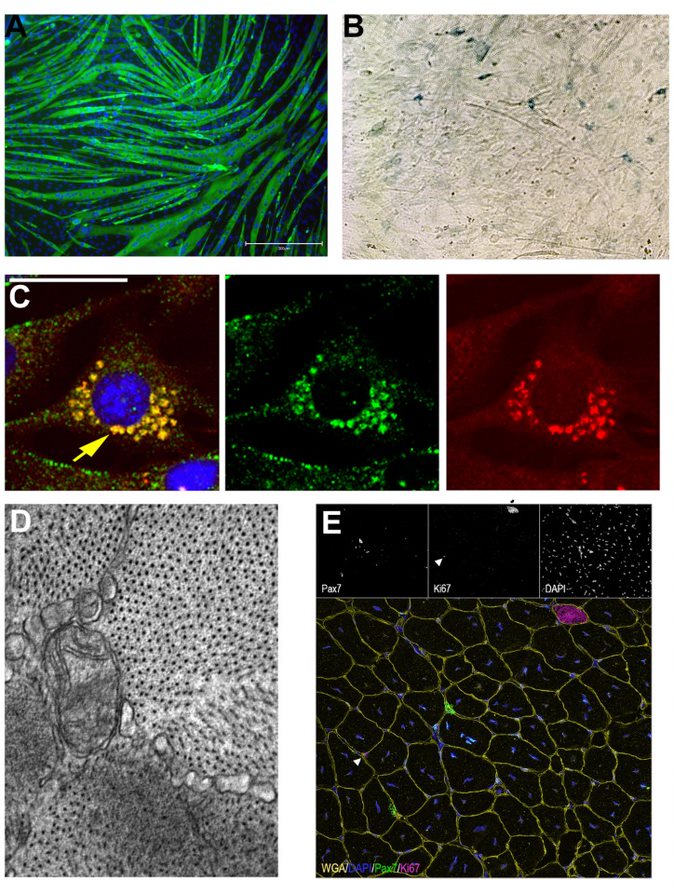
Figure 1. A. MF20 (green) staining of C2C12 myotubes. B SA-B-Gal staining showing senenscent primary mouse myoblasts. C. C2C12 myoblasts treated with Bafilomycin show stalled mitophagy as demonstrated by the presence of both green and red (mitoQc). D. Electron microscopy of a transverse myofiber showing mitochondira with disrupted cristae. E. WGA, Pax7 (satellite cells) and Ki67 (proliferation) staining of a transverse section of an injured mouse gastrocnemius.
Please see here for research publications.
Funding
- Irish Research Council (IRC)
- Science Foundation Ireland (SFI)
- Health Research Board (HRB)
- Orthopaedic Research UK