-
Courses

Courses
Choosing a course is one of the most important decisions you'll ever make! View our courses and see what our students and lecturers have to say about the courses you are interested in at the links below.
-
University Life

University Life
Each year more than 4,000 choose University of Galway as their University of choice. Find out what life at University of Galway is all about here.
-
About University of Galway

About University of Galway
Since 1845, University of Galway has been sharing the highest quality teaching and research with Ireland and the world. Find out what makes our University so special – from our distinguished history to the latest news and campus developments.
-
Colleges & Schools

Colleges & Schools
University of Galway has earned international recognition as a research-led university with a commitment to top quality teaching across a range of key areas of expertise.
-
Research & Innovation

Research & Innovation
University of Galway’s vibrant research community take on some of the most pressing challenges of our times.
-
Business & Industry

Guiding Breakthrough Research at University of Galway
We explore and facilitate commercial opportunities for the research community at University of Galway, as well as facilitating industry partnership.
-
Alumni & Friends

Alumni & Friends
There are 128,000 University of Galway alumni worldwide. Stay connected to your alumni community! Join our social networks and update your details online.
-
Community Engagement

Community Engagement
At University of Galway, we believe that the best learning takes place when you apply what you learn in a real world context. That's why many of our courses include work placements or community projects.
Shen Group
Introduction and Overview
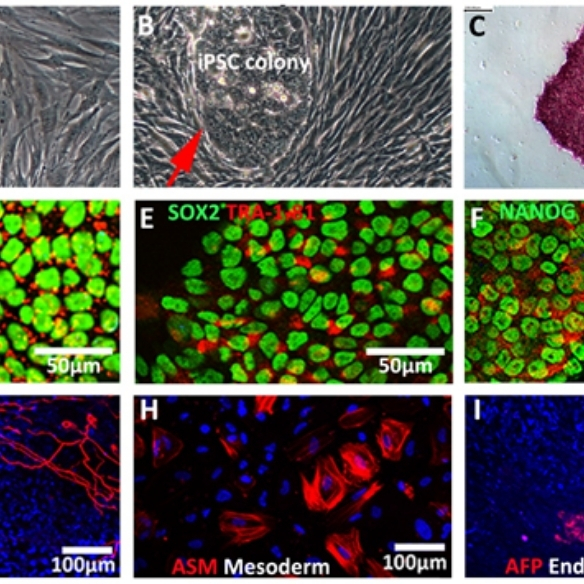
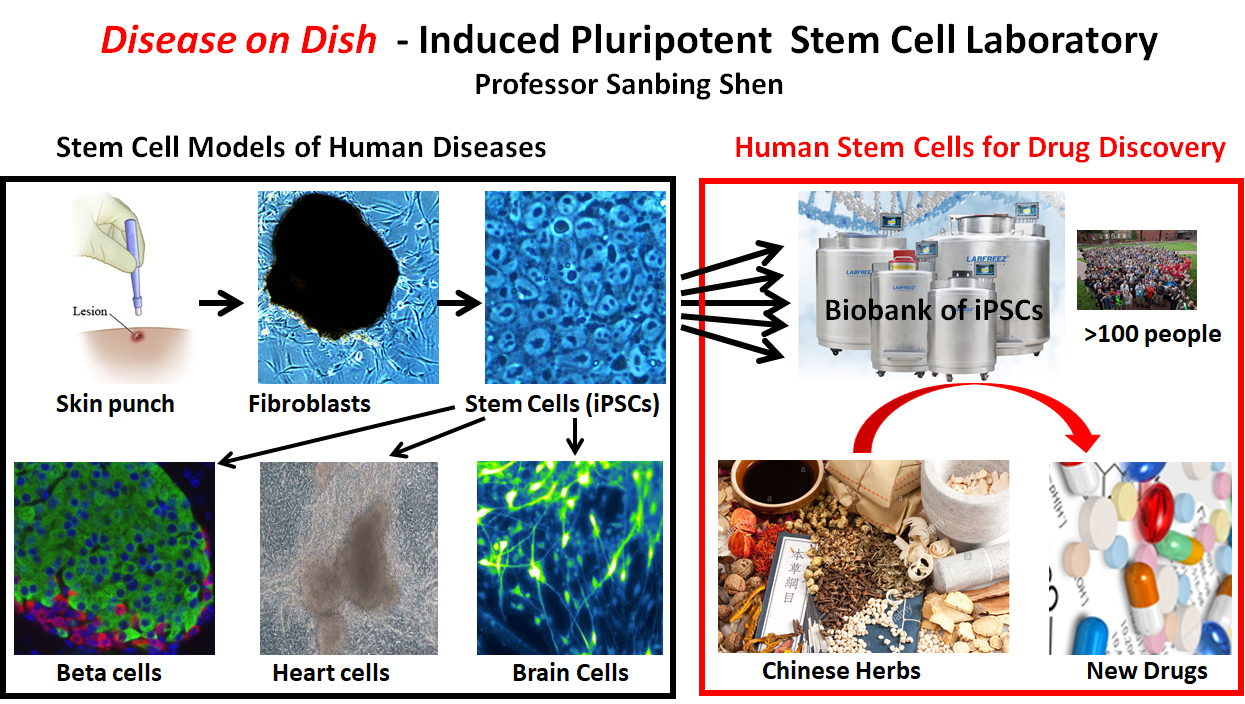
The Shen’s laboratory at NUI Galway focuses on stem cell modelling of the human diseases. Induced pluripotent stem cell (iPSC) technology developed by Nobel Laureate Yamanaka offers an unprecedented opportunity to create human disease models. Shen’s group has derived a biobank of human iPSCs from dermal fibroblasts of >100 volunteers, including healthy controls and patients with autistic spectrum disorders (ASD), epilepsy, amyotrophic lateral sclerosis (ALS), retinitis pigmentosa (RP) and long QT syndrome (LQTS). The iPSCs are differentiated into neurons and cardiomyocytes to investigate disease phenotype and mechanisms, as well as drug screening.
A biobank of iPSCs at NUI Galway for disease modelling and drug discovery.
The research have been funded by Science Foundation Ireland IvP, (SFI), SFI FutureNeuro Centre, Children's Health Foundation Temple Street, Irish Research Council (IRC), National Children’s Research Centre (NCRC), and Galway University Foundation (GUF). The current research areas include:
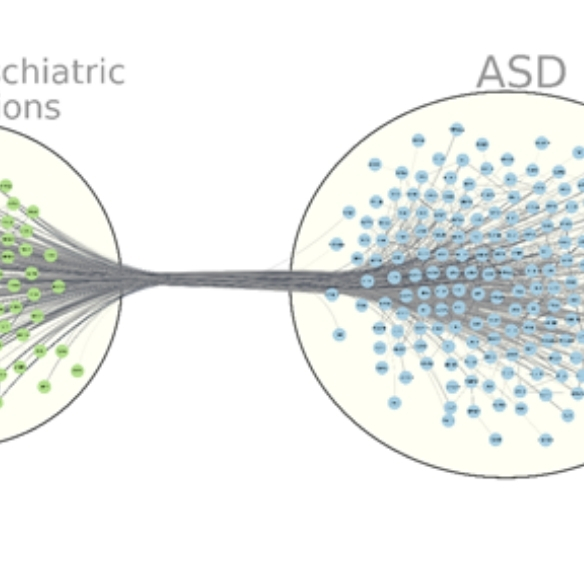
NRXN1 deletion and ASD (Funded by SFI, GUF)
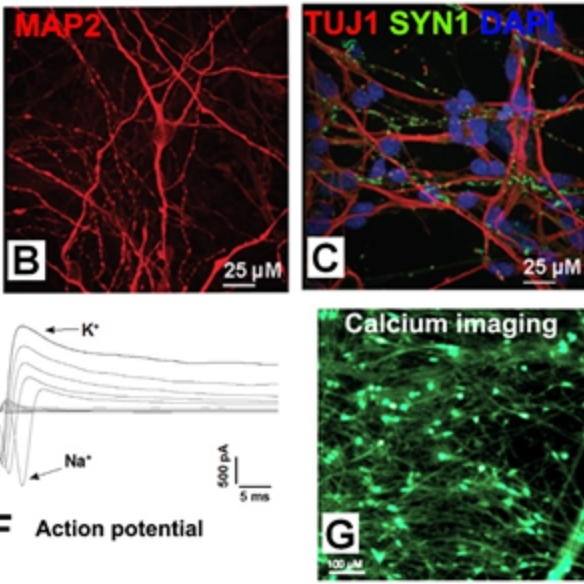
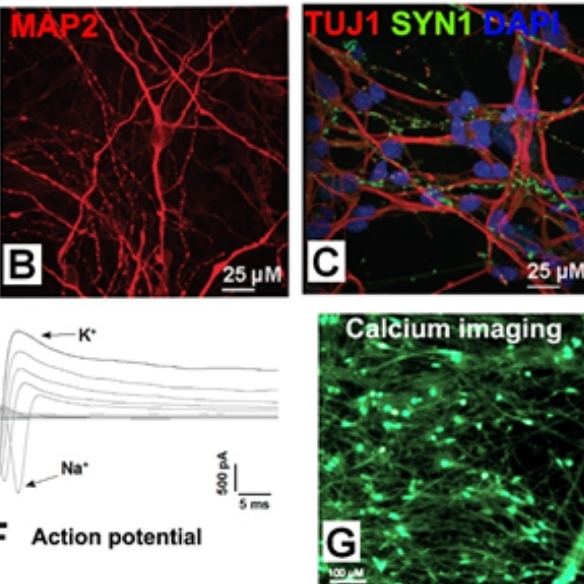
ASD is a devastating neurodevelopmental disorder and NRXN1 deletion is a major rare risk factors. In collaboration with Prof. Louise Gallagher at TCD and other scientists, controls and ASD patients with NRXN1+/- were recruited. Individual and familial NRXN1+/- iPSCs were derived and characterised. Isogenic NRXN1+/- iPSC lines are also generated using CRISPR/Cas9. The iPSCs are differentiated into 100-day excitatory cortical neurons, and analysed by calcium imaging, patch clamp, MEA and RNASeq. Bioinformatic analyses are carried out for related ASD pathways.
iPSC modelling for epilepsy (Funded by NUIG, IRC/SFI FutureNeuro, Temple Street Foundation)
ASD and epilepsy have approx. 30% mutual comorbidity. In collaboration with Prof. Nicky Allen at Galway University Hospital, sibling controls and patients with epilepsy and mutations on potassium voltage-gated ion channels (KCNA2, KCNQ2) were recruited. iPSCs are derived, and isogenic lines are being generated using CRISPR/Cas9. They are being differentiated into excitatory neurons and phenotyped by calcium imaging and MEA.
ALS and iPSC research (Funded by SFI FutureNeuro Centre)
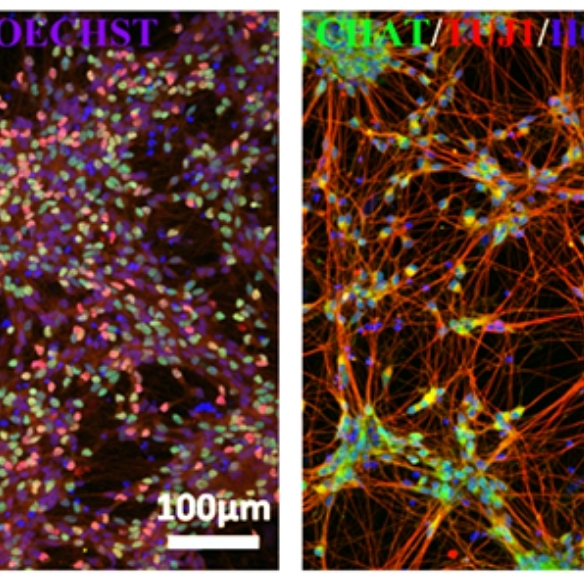
ALS is a neurodegenerative disorder and sporadic ALS is the majority. There is no effective treatment and most patients die within 3-5 years of diagnosis. In collaboration with Prof. Orla Hardiman at TCD, iPSCs were generated from healthy controls and sporadic ALS patients, and differentiated into motor neurons to compare MN differentiation, viability, and function via autophagy and MEA.
LQTS and iPSC research (Funded by NUIG, IRC, NCRC)
LQTS is an inherited cardiac ion channelopathy, and associated with ventricular arrhythmia and risk of sudden death. LQT1, LQT2 and LQT3 constitute the majority of LQTS and are caused by mutations on KCNQ1, KCNH2 and SCN5A genes, respectively. In collaboration with Dr Terence Prendiville and Prof. Timothy O’Brien, siling controls and LQT1/2/3 patients with defined mutations were recruited, and iPSCs and isogenic iPSC lines are created. They are differentiated into beating cardiomyocytes, and analysed by MEA and drug response.
Building drug screening platform.
Human proteins are subtly different from animal ones, and human iPSCs and derived cells are therefore vital for early step of drug screening and validation. We have established a specialized Confucius Institute of Chinese and Regenerative Medicine at NUI Galway, and one of the aims is to identify effective components in Chinese medicine using reporter iPSC lines. Libraries of Chinese herbs and small molecules will be screened, and lead compounds will be tested for efficacy and toxicity to develop combinatorial therapy of Chinese and regenerative medicine.
Animal modelling of human conditions.
Copy number variations have been associated with neurodevelopmental and neuropsychiatric disorders. Shen was previously trained on micromanipulation in China (Prof. De-Yu Lu and Miao Du), molecular biology and stem cells in Utrecht (Prof Siegfried de Laat, Christine Mummery, Paul van der Saag, Wiebe Kruijer) and artificial chromosomes in Edinburgh (Nick Hastie and Andreas Schedl), and utilised the knockout technology to investigate loss-of-function and overexpression using artificial chromosomes to model gain-of-function, which led to the following discoveries:
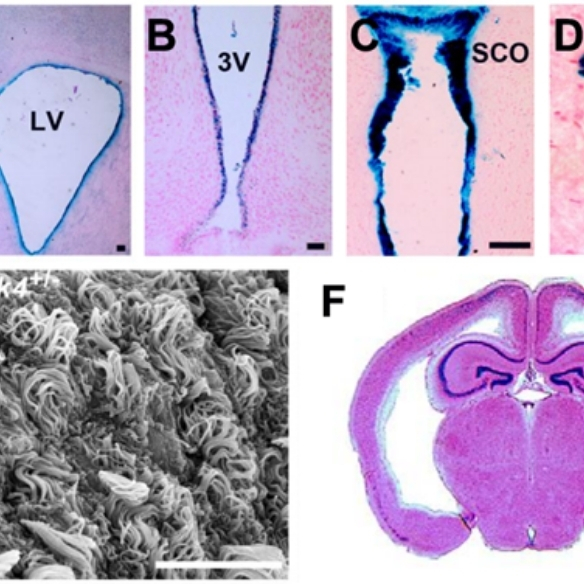
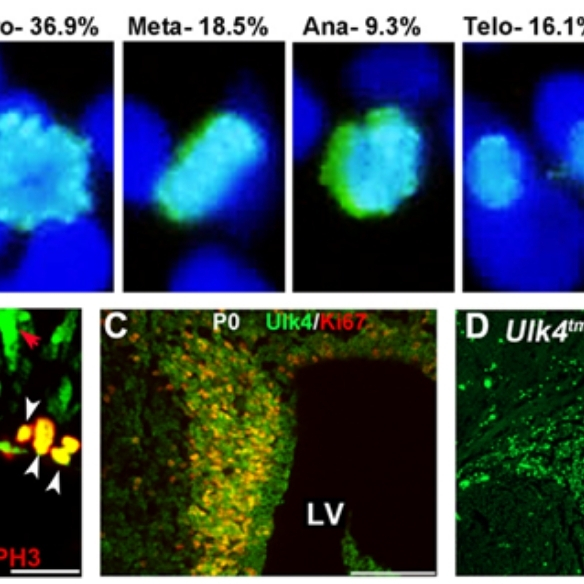
ULK4, a Ser/Thr kinase gene, is a rare risk factor for neurodevelopmental disorders (Lang et al, J Cell Sci. 2014;127:630-40), and its targeted deletion is associated with increased anxiety (Liu et a., Transl. Psych. 2018;8:43); reduced neural stem cell pool (Liu et al, Stem Cells 2016;34:2318-31), reduced myelination (Liu et al, GLIA 2018;66:175-90), aberrant ciliogenesis and hydrocephalus phenotype (Liu et al, J Neurosci. 2016;36:7589-600);
PAC1R, a G-protein coupled receptor for neuropeptide PACAP, is requiredfor ciliary function, and PAC1R overexpression from a 130kb PAC leads to increased PKA/PKC activity and hydrocephalus-like phenotype in mice (Lang et al, J. Clin. Invest. 2006;116:1924-34). These studies showed that the Ser/Thr kinase activity in ventricular ependymal layers can be the drug target for treating ciliary disorders and hydrocephalus;
VIPR2, encoding a G-protein coupled receptor for neuropeptide VIP, is a master switch of circadian clock. Overexpression of human VIPR2 in a 550kb YAC alters circadian function in mice (Shen et al, PNAS2000;97:11575-80), and targeted deletion of endogenous Vipr2 leads to the loss of the circadian clock and jetlag (Harmar et al, Cell2002;109:497-508), suggesting VPAC2 receptor can be an effective drug target for sleeping disorders and jetlag;
5-HTT, encoding serotonin transporter, is associated with mood and unexpected pulmonary function, and overexpression of human 5-HTT from a 500kb YAC vector leads to pulmonary arterial hypertension in transgenic mice (MacLean et al, Circulation 2004;109:2150-5) and altered anxiety (Jennings et al, J. Neurosci. 2006;26:8955-64) ;
Overexpression of truncated Disc1 from a 148kb BAC transgene is associated with schizophrenia-like phenotype in transgenic mice (Shen et al., J. Neurosci. 2008;28:10893-904), offering a mouse model for schizophrenic research.
Please see here for research publications.
Funding
- Science Foundation Ireland (SFI)
- Enterprise Ireland (EI)
- Galway University Foundation (GUF)
- China Scholarship Council (CSC)
- Temple Street Foundation