-
Courses

Courses
Choosing a course is one of the most important decisions you'll ever make! View our courses and see what our students and lecturers have to say about the courses you are interested in at the links below.
-
University Life

University Life
Each year more than 4,000 choose University of Galway as their University of choice. Find out what life at University of Galway is all about here.
-
About University of Galway

About University of Galway
Since 1845, University of Galway has been sharing the highest quality teaching and research with Ireland and the world. Find out what makes our University so special – from our distinguished history to the latest news and campus developments.
-
Colleges & Schools

Colleges & Schools
University of Galway has earned international recognition as a research-led university with a commitment to top quality teaching across a range of key areas of expertise.
-
Research & Innovation

Research & Innovation
University of Galway’s vibrant research community take on some of the most pressing challenges of our times.
-
Business & Industry

Guiding Breakthrough Research at University of Galway
We explore and facilitate commercial opportunities for the research community at University of Galway, as well as facilitating industry partnership.
-
Alumni & Friends

Alumni & Friends
There are 128,000 University of Galway alumni worldwide. Stay connected to your alumni community! Join our social networks and update your details online.
-
Community Engagement

Community Engagement
At University of Galway, we believe that the best learning takes place when you apply what you learn in a real world context. That's why many of our courses include work placements or community projects.
Microscopy
Micron-sized Test Tubes
The Nanoscale Biophotonics Laboratory has been developing one of the most advanced fluorescence microscopy facilities in Ireland. We currently operate four confocal and one TIRF systems (4 fluorescence and 1 Raman). These systems are used for a variety of materials science and biological applications. We generally use these systems for quantitative spectroscopic analysis rather than imaging. The microscopes provide us with micron- and nanometre- sized analysis volumes (i.e. test tubes) with which to probe and measure a variety of molecular processes.
These are all located in the School of Chemistry in a dedicated laboratory (see pictures below).
 |
Inverted FLIM-FCS:
In April 2005 the inverted Fluorescence Lifetime Imaging Microscopy (FLIM) and Fluorescence Correlation Spectroscopy (FCS) facility was delivered to the laboratory, funded by a Health Research Board Equipment grant. The FLIM system is based around the Alba system from ISS. The Alba is a confocal microscope fitted with a variety of excitation sources, full imaging capability, and the provision for a variety of different single molecule detection experiments including FCS and Fluorescence Cross Correlation Spectroscopy (FCCS).
The picture below shows the inverted microscope version which uses an Olympus IX71 frame and is now being optimized for protein analysis studies.
 ( July. 2016 spec.) ( July. 2016 spec.) This ISS system is dual emission channel confocal microscope which is capable of undertaking a wide variety of Single Molecule Detection (SMD) experiments. In July 2016 it was upgraded to a photon counting based gfacility by ISS and now uses the Becker & Hickl SPC150 TCSPC module for both FCS and FLIM applications. A variety of pulsed laser diodes can be fibre coupled into the system and it is currently configured for 405 and 470 nm excitation. The system electronics and software was also upgraded. The new system can accommodate any pulsed visible wavelength excitation source and will make use of the new supercontinuum laser source which is also available. Additional Functionalities: It is equipped with a microscope incubator from Solent Scientific. Funding for this enhancement was provided under the HEA funded (PRTLI-IV) National Biophotonics Imaging Platform. The chamber is rated for operation between 32C and 40C. The system is also equipped with an Electron Multiplying CCD (EMCCD) Camera for ultra-low light imaging. The EMCCD is an Andor Luca system which we are using for low-light sample imaging. |
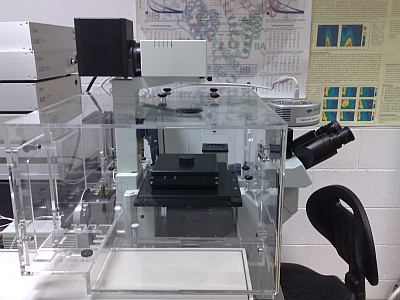  |
| (2005 Picture, original spec.) The original system had 470 & 532 nm excitation sources to enable 2 channel cross correlation experiments to be conducted (Biochemistry Dept. projects with HP Nasheuer and Ian Dobbie). The system had two seperate detectors channels allowing four dual colour imaging, a motorized XYZ stage (with nm resolution), and a range of high performance objectives. The microscope objectives are also compatible with our existing fluorescence and Raman microscopy systems. In 2007 the system was upgraded with a fibre coupled laser delivery system. |
Some sample data from the inverted system is illustrated below:
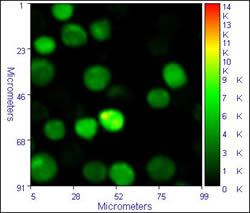 |
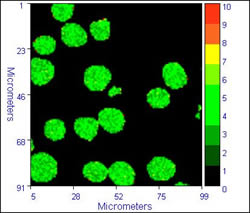 |
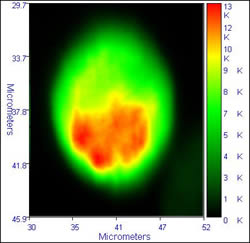 |
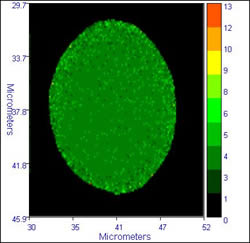 |
The images on the left show typical fluorescence intensity images of dichlorofluorescein labelled cells, while the images on the right dhow the FLIM images (lifetime by modulation) of the cells. The calculated lifetime in the cells is a near uniform 3.6 /- 0.4 ns. The images were accumulated with a residence time of 1ms/pixel, 470 nm excitation, and an emission filter cantered at 520 nm. These images show to good advantage the ability of FLIM to overcome intensity fluctuations and give a truer image of what happens in the cells
Cells provided by Dr. Michael Ball, ex. of NCBES, who was using FLIM to study cell biomaterial interactions.
Upright FLIM-FCS
The upr ight FLIM microscope for materials science and photophysics applications. The system became operational in June 2005 and is based on the Alba system from ISS. ight FLIM microscope for materials science and photophysics applications. The system became operational in June 2005 and is based on the Alba system from ISS. It comprises of an upright Olympus microscope, equipped with both a micrometer stepper motor and piezo nanopositioning stage. The detector system has two independent channels for dual colour FLIM imaging, and dual colour FCS. The excitation sources are 405 & 635 nm modulated laser diodes, as well as an epifluorescence illuminator for wide field imaging. |
We used this system for measuring:
- Photophysical properties of fluorophore doped biomedical polymers,
- Emission properties of Hydrocarbon bearing Fluid Inclusions (HCFI),
- Emission properties of crude petroleum oils,
- Photophysics of a novel class of fluorophore synthesized in NUI-Galway.
This system was funded by an equipment grant from Science Foundation Ireland through the supplemental equipment grant scheme.
Total Internal Reflection fluorescence microscopy (TIRFM)
 An Olympus cell^tool TIRF microscope is also available in-house. The system has three laser excitation sources (405, 488, and 561 nm), standard white light illumination (MT20) for conventional imaging, and a low light Andor EMCCD camera for ultra-low light imaging. This system was funded by Science Foundation Ireland under their equipment grants scheme in 2007.
An Olympus cell^tool TIRF microscope is also available in-house. The system has three laser excitation sources (405, 488, and 561 nm), standard white light illumination (MT20) for conventional imaging, and a low light Andor EMCCD camera for ultra-low light imaging. This system was funded by Science Foundation Ireland under their equipment grants scheme in 2007.
The triple line TIRF system is located in Lab166. The Andor EMCCD Camera is on the left, the TIRF illuminators at rear of microscope. This has also been modified with an Ocean Optics fluorescence spectrometer attached to the right hand side port to enable spectroscopic measurements.
We are currently developing fluorescence based methods for the analysis of protein-surface interactions using this system.
 |
 |
| Left rear of TIRF setup, showing 488 nm laser input via single mode FC fibre. The micrometer screw allows for adjustment of laser input angle. | Right rear of TIRF setup, showing 405 nm laser input via single mode FC fibre. The micrometer screw allows for adjustment of laser input angle. The oblong box is the laser safety shutter. |
Fibre Optic Confocal Lifetime Microscope:
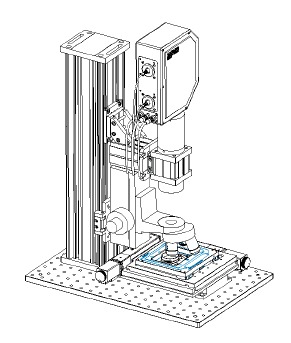 A confocal scanning lifetime microscope system is to be constructed for materials analysis using TCSPC measurement methods. A confocal scanning lifetime microscope system is to be constructed for materials analysis using TCSPC measurement methods. It will utilize existing components and accommodate the humidity chamber and eventually a heating-freezing stage. This system used a PicoQuant PicoHarp300 TCSPC measurement system, pulsed laser diode excitation, and a ThorLabs VCM100 Confocal scanner (shown left). This will allow for FLIM and FCS measurements to be undertaken. We will use this system for exploratory studies in materials science, particularly for polymer systems and metal enhanced fluorescence studies. The system was delivered in May 2008. It is fitted with a dual laser source (405 and 635 nm) for conventional fluorescence imaging. This facility was provided under the HEA funded (PRTLI-IV) National Biophotonics Imaging Platform. |
 |
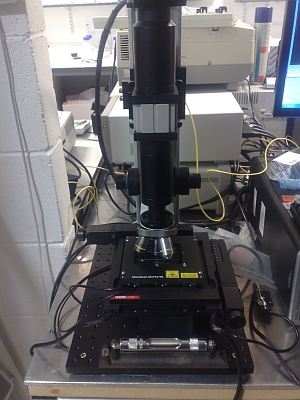 |
| The image above shows the complete system with PC control. The scan unit is at the top with two yellow single mode fibres attached. The two boxes immediately to the rear accommodate the lasers, detectors, and control electronics. | This close up shows the basic construction of the LSCM with a motorised stage and a relatively open optical path suitable for easy modification. The stage is PC controlled with XYZ travel at sub-micron resolution. |
Sampling Systems:

A VGI 2000M custom built humidity chamber (right) for microscopy and spectroscopy was commissioned in Jan. 2008. This is heavily used for polymer studies. The system can be fitted to most of the Raman or Fluorescence microscopy systems. We have also acquired a CO2 perfusion system and chamber for live cell imaging which can be fitted to the Inverted FLIM-FCS system. We also have a variety of cell and sample holders for static measurements. As we acquire more equipment and accessories I will update the webpages with details and photos. |
Historical systems:
A microscope based system for fluorescence lifetime measurement (lifetimes < 0.3 ns, resolution <10 microns) was built as a Ph.D. research project by two students (M. Przyjalgowski and B. Szczupak) and was used for the study of fluorophore labeled polymer films, nanoparticle coated microspheres, and microscopic hydrocarbon bearing fluid inclusions. Funding for this project came from the National Centre for Biomedical Engineering Science.
 The picture (left) shows the microscope system in operation. The excitation source, coupling optics, and detection system were all mounted above the microscope (as shown in the lower picture), in a light tight enclosure that can be easily opened for alignment. The major system components are a pulsed 404 nm laser diode (PicoQuant), an Olympus BX-60 microscope, a 0.10 m focal length monochromator (Scientech 9030) controlled by STP-240 stepper motor controller. The detector was a Hamamatsu H5783P-01 photon counting PMT module and the output of which was fed to a SPC-730 TCSPC card integrated into a standard PC. The picture (left) shows the microscope system in operation. The excitation source, coupling optics, and detection system were all mounted above the microscope (as shown in the lower picture), in a light tight enclosure that can be easily opened for alignment. The major system components are a pulsed 404 nm laser diode (PicoQuant), an Olympus BX-60 microscope, a 0.10 m focal length monochromator (Scientech 9030) controlled by STP-240 stepper motor controller. The detector was a Hamamatsu H5783P-01 photon counting PMT module and the output of which was fed to a SPC-730 TCSPC card integrated into a standard PC. |
|
|
|
|
ICCD-FLIM:
 This system was built by a PhD student, Richard Murray. This system was built by a PhD student, Richard Murray. The system used a fast gated ICCD camera from Stanford Computer Optics to generate time-gated images from which Fluorescence Lifetime Images can be constructed. The Camera is a 4 Picos which is capable of producing a minimum time gate of about 200 picoseconds. The excitation source is a PicoQuant 405 nm pulsed laser diode. This was used for FLIM analysis of Hydrocarbon Bearing Fluid Inclusions. This has now been retired from use. |
Various Pics of the microscope lab.
   |
  |
| This is what lab 166 looked like before we moved everything in. | This is the inverted FLIM-FCS system which has just been moved into it's new room. It was reassembled by the postdocs and PhD students. |
 |
 |
| R166 lab, late 2008: Upright FLIM system | R166 lab, late 2008: TIRF system. |
 |
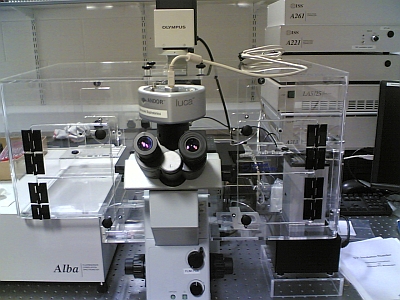 |
| R166 lab, late 2008: Raman & VCM100 confocal | NCBES lab, 2006?: Inverted FLIM/FCS system. |








 The laser source is at the bottom left, most of the package contains the peltier cooler which keeps the diode at an operating temperature of ~20° C for stable wavelength and power output. The PMT detector is mounted onto the exit port of the monochromator (top). This type of system requires practically no modification whatsoever to the microscope and in principle could be attached to most laboratory microscopes without any difficulty. The system is currently located in R307.
The laser source is at the bottom left, most of the package contains the peltier cooler which keeps the diode at an operating temperature of ~20° C for stable wavelength and power output. The PMT detector is mounted onto the exit port of the monochromator (top). This type of system requires practically no modification whatsoever to the microscope and in principle could be attached to most laboratory microscopes without any difficulty. The system is currently located in R307.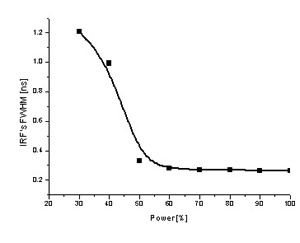 The plot on the left shows how the temporal width (FWHM) of the Instrument Response Function (IRF) varies with the laser power. For the LDH-400 violet laser diode, lasing does not occur until the power setting on the dial exceeds ~ 30%, this results in a broad ASE and hence a large FWHM value. The FWHM drops rapidly as lasing increases and above ~ 60% power the IRF remains nearly constant at ~270 picoseconds. This value is the limit of the system and is determined by the detector which is an uncooled PMT and as such is not capable of better temporal resolution. To improve the temporal resolution further we need a MCP (Multichannel Plate) detector which would allow us to reduce the FWHM to ~ 70 picoseconds, which is the temporal width of the laser pulses. We also developed a motorised X-Y scanning stage for the DLFLM. In 2005, we replaced the DLFLM with dedicated FLIM systems (see Alba above). The microscope frame and components from the DLFLM were recycled to build a gated ICCD-FLIM system and a fibre coupled TCSPC module for TRES and lifetime studies.
The plot on the left shows how the temporal width (FWHM) of the Instrument Response Function (IRF) varies with the laser power. For the LDH-400 violet laser diode, lasing does not occur until the power setting on the dial exceeds ~ 30%, this results in a broad ASE and hence a large FWHM value. The FWHM drops rapidly as lasing increases and above ~ 60% power the IRF remains nearly constant at ~270 picoseconds. This value is the limit of the system and is determined by the detector which is an uncooled PMT and as such is not capable of better temporal resolution. To improve the temporal resolution further we need a MCP (Multichannel Plate) detector which would allow us to reduce the FWHM to ~ 70 picoseconds, which is the temporal width of the laser pulses. We also developed a motorised X-Y scanning stage for the DLFLM. In 2005, we replaced the DLFLM with dedicated FLIM systems (see Alba above). The microscope frame and components from the DLFLM were recycled to build a gated ICCD-FLIM system and a fibre coupled TCSPC module for TRES and lifetime studies. nuigalway.ie
nuigalway.ie 







