-
Courses

Courses
Choosing a course is one of the most important decisions you'll ever make! View our courses and see what our students and lecturers have to say about the courses you are interested in at the links below.
-
University Life

University Life
Each year more than 4,000 choose University of Galway as their University of choice. Find out what life at University of Galway is all about here.
-
About University of Galway

About University of Galway
Since 1845, University of Galway has been sharing the highest quality teaching and research with Ireland and the world. Find out what makes our University so special – from our distinguished history to the latest news and campus developments.
-
Colleges & Schools

Colleges & Schools
University of Galway has earned international recognition as a research-led university with a commitment to top quality teaching across a range of key areas of expertise.
-
Research & Innovation

Research & Innovation
University of Galway’s vibrant research community take on some of the most pressing challenges of our times.
-
Business & Industry

Guiding Breakthrough Research at University of Galway
We explore and facilitate commercial opportunities for the research community at University of Galway, as well as facilitating industry partnership.
-
Alumni & Friends

Alumni & Friends
There are 128,000 University of Galway alumni worldwide. Stay connected to your alumni community! Join our social networks and update your details online.
-
Community Engagement

Community Engagement
At University of Galway, we believe that the best learning takes place when you apply what you learn in a real world context. That's why many of our courses include work placements or community projects.
Duffy Group
Current research focuses on advanced biomaterials, cell-based therapies and implantable therapeutic reservoirs as treatments for chronic diseases. The lab is funded by Enterprise Ireland, Science Foundation Ireland and the European Union’s Horizon 2020 programme. To date, research from the Duffy lab has resulted in a range of transformative technologies that allow for therapeutic agents to be deployed in a precise, safe, minimally invasive and maximally efficacious manner. Complex health challenges demand sophisticated solutions and new thinking. Poor efficacy and unacceptable side-effects are largely a result of simplistic delivery techniques that do not meet these challenges. Rather, bioinspiration and synergistic combinations of advanced materials and techniques are required to shift paradigms and inform meaningful therapeutic breakthroughs. This ethos has allowed the Duffy lab to develop a suite of advanced technologies that pave the way for enhanced patient outcomes. Through leading the AMCARE, DRIVE and DELIVER H2020 programmes the Duffy Lab has developed:
- Implantable reservoirs for a range of disease indications for local delivery and replenishment of therapeutic cargos. These devices have been scaled to clinical size for long-term testing in large animals. We have also invented delivery devices that permit minimally invasive deployment while using large cohort imaging databases to define population fit designs for future clinical translation. Technologies from the lab have been assigned/licenced to Boston Scientific and Reverse1 Therapeutics.
- Injectable hydrogel systems in combination with stem cells or insulin producing cells to promote post-infarct regeneration or normoglycemia in diabetes. Such hydrogels have incorporated advanced drug delivery features, such as on-demand and multimodal delivery, and have been scaled to GMP manufacture with SOPs and methods currently used across 5 sites in Europe (Oxford, NMI, Explora, Abiel and Trinity College Dublin) and 6 sites in the US (MIT, UWash, Georgia Tech). Delivery devices and catheters have also been developed and tested in large animal models.
The lab is actively involved in two SFI research centres (CURAM and AMBER). In AMBER, the labs work is under the Materials for Health Platform where the group will continue to develop novel biomaterial carriers for delivery of cell-based therapeutics to treat cardiovascular disease and diabetes. In CURAM, researchers from the lab are developing advanced delivery devices for complex biomaterial-based therapies for cardiac repair.
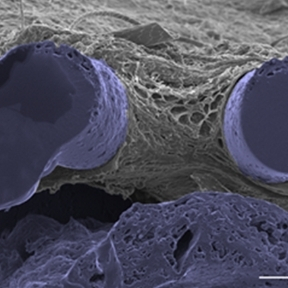
Implantable Reservoirs and Soft Robotics
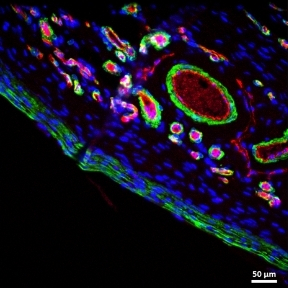
The performance of indwelling medical devices that depend on an interface with soft tissue is plagued by complex, unpredictable foreign body responses. Such devices—including breast implants, biosensors, and drug delivery devices—are often subject to a collection of biological host responses, including fibrosis. Fibrosis of soft tissue surrounding these devices can often impair their functionality and cause the devices to become ineffective.
The Duffy Lab works on the design and development of novel implantable soft robotic reservoirs which modulate and withstand the foreign body response, improving functionality and durability of implantable medical devices. This includes the development of a milliscale actuatable dynamic soft reservoir (DSR) that actively modulates the biomechanics of the biotic-abiotic interface by altering tissue dynamics and cellular activity within the implant tissue. Our studies have found that the actuated DSR shows a significant reduction in fibrous capsule formation and myofibroblasts surrounding the device (1). These actuatable DSR device have potential applications in the treatment of Type 1 diabetes.
Biomaterials
According to the WHO, ischemic cardiovascular disease is the world’s leading cause of death (2). Advanced cell therapies have been shown to be minimally effective likely due to poor retention and survival of the cells in the cardiac tissue. This has led to the use of biomaterials to deliver cells to the myocardium with increased retention rate.
Professor Duffy’s research includes the development of an Advanced Material Catheter (AMCath) that overcomes translational hurdles associated with delivering hyaluronic acid hydrogels to the myocardium. We have also shown AMCath can be used to deliver cardiopoietic adipose-derived stem cell-loaded hydrogels without compromising the viability (80% viability) of the cells in vitro. These developments offer a potentially new clinical strategy to repair damaged myocardium and improve cell viability after infusion (3).
Drug Delivery
Approximately 1.5 million cases of myocardial infarction (MI) occur annually in the United States alone. Although the 30 day mortality rate post-MI has reduced significantly in the past two decades, it still remains at 7.8% (4). Post-MI complications often include complex alterations to the myocardial tissue known as ventricular remodelling. Current therapies for the treatment of post-MI complications are not always effective long-term. Improvements in cell and drug delivery methods offer promising novel methods of cardiotherapeutic and cardiorestorative treatments.
Alongside colleagues in Trinity College Dublin, Royal College of Surgeons Ireland and AMBER, Professor Duffy has been a lead investigator in many research projects which aim to improve the treatment of MI, ischemic heart disease and Type 1 diabetes. These projects include the development of hydrogels for the delivery of pharmaceutical and regenerative therapies. These novel hydrogels are capable of significantly enhancing the metabolic activity of dermal fibroblasts (5).
Increasing vascularisation of the affected myocardial area has also been shown to reduce patient symptoms. The growth factors required to do this have short half-lives making development of an efficacious therapy difficult. Current research on the development of a nanomedicine-loaded hydrogel for sustained delivery of growth factors to the ischaemic myocardium offers a prototype for a novel delivery system of cardiorestorative treatments for millions of patients annually (6).
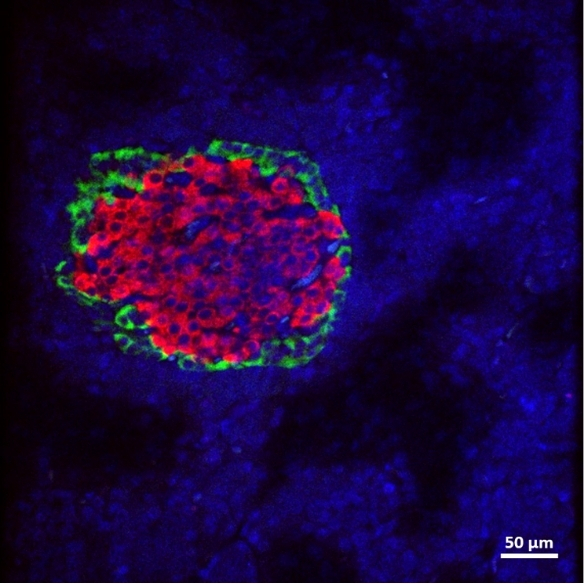
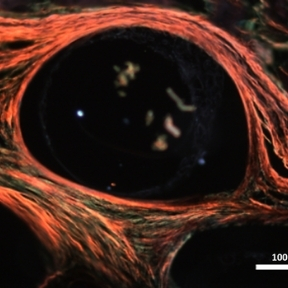
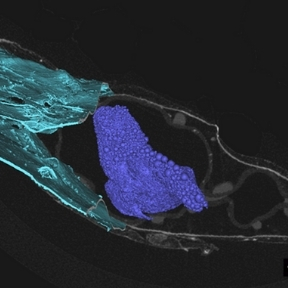
Macroencapsulation of Pancreatic Islet Cells
Professor Duffy leads the DRIVE and DELIVER projects which aim to improve pancreatic islet transplant therapy for diabetes mellitus. The use of islet transplantation for the treatment of Type 1 diabetes (T1D) is severely hampered by a number of factors including the shortage of donor tissue, low cell-survival, and the need for life-long immunosuppression. The Duffy Lab is a leading force in the research and development of islet cell macroencapsulation devices. Macroencapsulation devices provide the possibility of immunoprotecting transplanted pancreatic islet cells improving cell viability and reducing the volume of islet cells needed. We have shown that macroencapsulated syngeneic islets can maintain glucose responsiveness and function for up to 8 weeks in large animal trials.
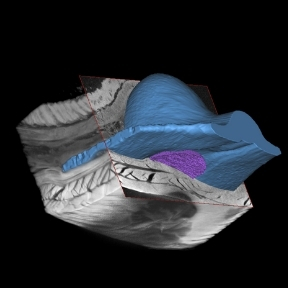
A number of anatomical sites have been investigated for suitability for the implantation of an islet macroencapsulation device. The Duffy Lab has identified the anterior abdominal wall as a location with adequate space and vascularisation which is easily surgically accessible while remaining minimally invasive.
Although a great deal of future work is required to translate these results from animal to clinical trials, these findings offer possible solutions to current limitations of islet transplantation therapies and macroencapsulation devices (7).
Please see here for research publications.
Funding
- Enterprise Ireland (EI)
- Science Foundation Ireland (SFI)
- European Union Horizon 2020 programmes
- Advanced Materials and BioEngineering Research Centre (AMBER)