-
Courses

Courses
Choosing a course is one of the most important decisions you'll ever make! View our courses and see what our students and lecturers have to say about the courses you are interested in at the links below.
-
University Life

University Life
Each year more than 4,000 choose University of Galway as their University of choice. Find out what life at University of Galway is all about here.
-
About University of Galway

About University of Galway
Since 1845, University of Galway has been sharing the highest quality teaching and research with Ireland and the world. Find out what makes our University so special – from our distinguished history to the latest news and campus developments.
-
Colleges & Schools

Colleges & Schools
University of Galway has earned international recognition as a research-led university with a commitment to top quality teaching across a range of key areas of expertise.
-
Research & Innovation

Research & Innovation
University of Galway’s vibrant research community take on some of the most pressing challenges of our times.
-
Business & Industry

Guiding Breakthrough Research at University of Galway
We explore and facilitate commercial opportunities for the research community at University of Galway, as well as facilitating industry partnership.
-
Alumni & Friends

Alumni & Friends
There are 128,000 University of Galway alumni worldwide. Stay connected to your alumni community! Join our social networks and update your details online.
-
Community Engagement

Community Engagement
At University of Galway, we believe that the best learning takes place when you apply what you learn in a real world context. That's why many of our courses include work placements or community projects.
Howard Group
Genetic modification of stem and progenitor cells
Stem cells and progenitor cells show great promise as treatments for diseases and repair of tissue damages. These cells are also useful tools to study developmental processes in a human system in vitro.
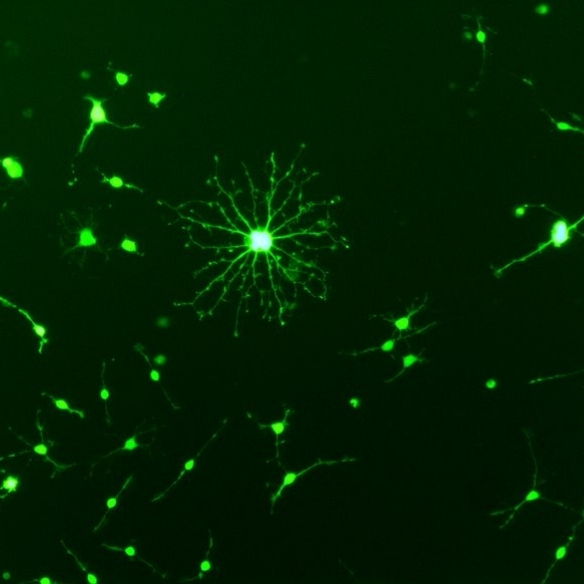
Our group is interested in genetically modifying stem cells to (i) to better understand how they function and (ii) to enhance their therapeutic potential. Using viral vectors we can efficiently overexpress proteins in cells (figure 1, overexpression of GFP in an oligodendrocyte progenitor cell), or we can deliver short hairpin RNAs (shRNAs) which reduce the levels of protein in cells (figure2 showing that 2 shRNAs reduced the production of NG2 by astrocytes).
Comparison of stem and progenitor cells from healthy volunteers and patients with diseases
Stem and progenitor cells can be used autologously ( where a patient is given their own cells) or allogeneically (cells are obtained from a donor). It is possible that cells from patients with chronic diseases and/ or older patients may not grow or function as well as cells from young healthy donors. We are studying cells from patients with conditions such as critical limb ischaemia and type II Diabetes Mellitus to determine how they compare to cells from healthy donors.
Ireland’s first clinical trial of an ex vivo expanded progenitor cell was conducted at NUI Galway and Galway University Hospital with GMP clinical grade cells produced at the Center for Cell Manufacturing Ireland (CCMI). Patients with ‘no option’ critical limb ischemia were given their own cells. While the therapy was safe we observed that it was more difficult to manufacture the required cell dose for older patients than for young healthy donors. We are continuing to determine how patient cells compare to healthy young cells and whether patient cells can be altered to enhance their function.
Genetic modification strategies to enhance recovery after spinal cord injury.
Spinal cord injury can have a devastating impact of a patient’s quality of life. This condition is the focus of research efforts all over the world.
Following spinal cord injury a glial scar is formed, initially this has a protective function, but it inhibits repair and regeneration of the damaged spinal cord. Our group, in collaboration with Dr Siobhan McMahon in Anatomy are developing gene therapy approaches to removing or / breaking down the glial scar. The efficacy of these approaches are tested in ex vivo organotypic cultures. (Figure 2) In addition we are evaluating strategies to provide factors such as neurotrophins which enhance repair and regeneration.