-
Courses

Courses
Choosing a course is one of the most important decisions you'll ever make! View our courses and see what our students and lecturers have to say about the courses you are interested in at the links below.
-
University Life

University Life
Each year more than 4,000 choose University of Galway as their University of choice. Find out what life at University of Galway is all about here.
-
About University of Galway

About University of Galway
Since 1845, University of Galway has been sharing the highest quality teaching and research with Ireland and the world. Find out what makes our University so special – from our distinguished history to the latest news and campus developments.
-
Colleges & Schools

Colleges & Schools
University of Galway has earned international recognition as a research-led university with a commitment to top quality teaching across a range of key areas of expertise.
-
Research & Innovation

Research & Innovation
University of Galway’s vibrant research community take on some of the most pressing challenges of our times.
-
Business & Industry

Guiding Breakthrough Research at University of Galway
We explore and facilitate commercial opportunities for the research community at University of Galway, as well as facilitating industry partnership.
-
Alumni & Friends

Alumni & Friends
There are 128,000 University of Galway alumni worldwide. Stay connected to your alumni community! Join our social networks and update your details online.
-
Community Engagement

Community Engagement
At University of Galway, we believe that the best learning takes place when you apply what you learn in a real world context. That's why many of our courses include work placements or community projects.
Solid-State API analysis
Solid-state analysis
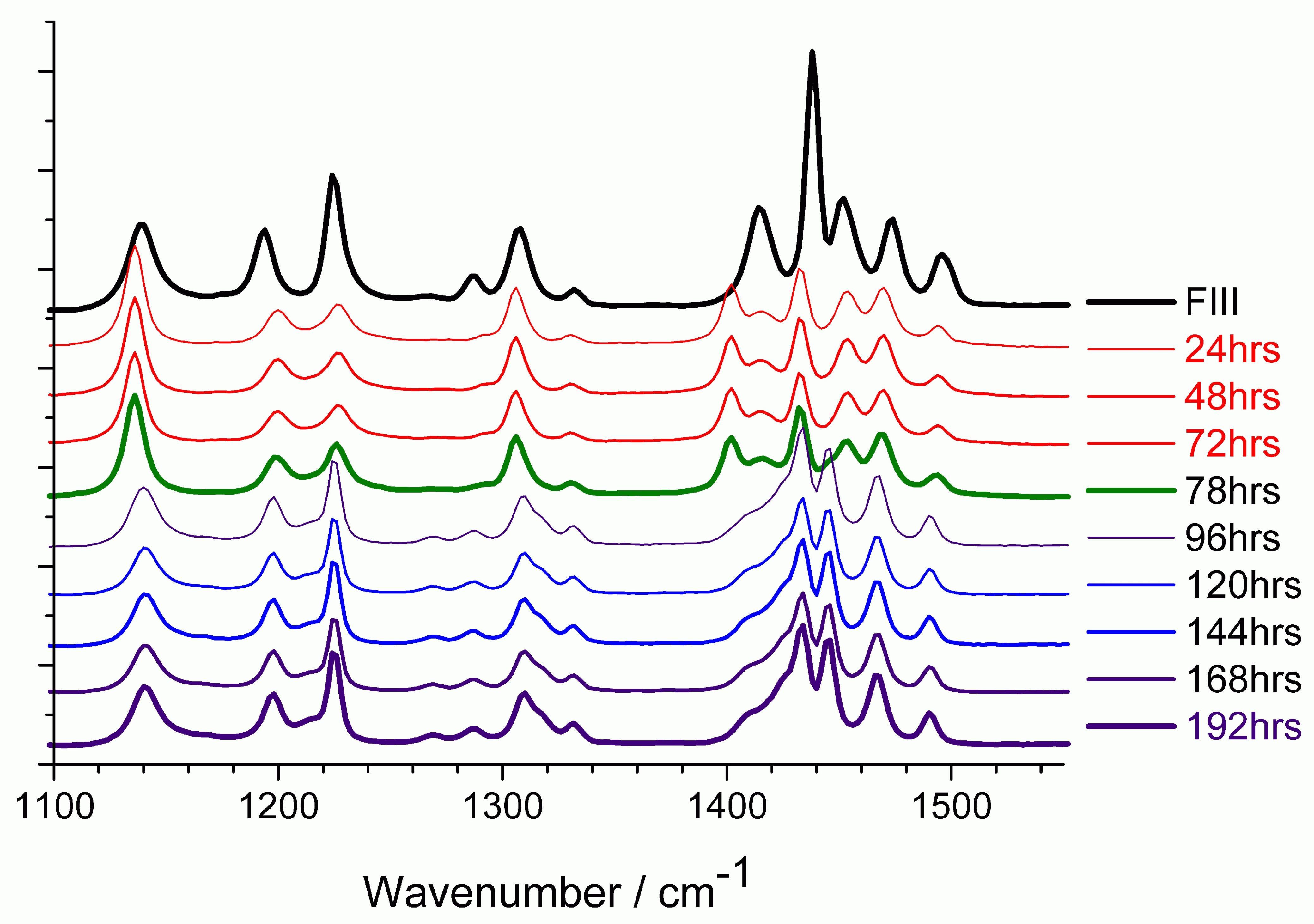
Real-Time-Release (RTR) and low-level polymorph and Impurity analysis of Active Pharmaceutical Ingredients (APIs): Part of the SFI funded Synthesis and Solid-State Pharmaceutical Centre. A vital issue associated with the manufacture and analysis of solid state APIs and their formulations is the detection and quantification of chemical contaminants and alternate API polymorphs, present at low concentrations. For the RTR of APIs one must validate the quality of the materials in terms of precisely defined contamination limits which is often accomplished by time-consuming methods like High-Performance Liquid Chromatography (HPLC) or other wet chemistry based methods. Being able to replace these traditional methods with on/in-line spectroscopic based methods will provide significant cost savings to the global PharmaChem sector. The research focuses on the use of Raman spectroscopy and novel chemometric methods. We have recently published the first technical method which demonstrated a Limit of Detection (LoD) of 0.03% for a binary powder mixture.
 |
|
| Raman spectra (backscattered) showing the conversion of piracetam form III to form II via transformation through FI. The top trace represents starting (FIII) material at room temperature, the 24–72 h traces are the sample at 140 ◦C (FI form), and the 78–192 h traces the samples at room temperature after heating. |
Publications:
- Chemometric approaches to low-content quantification (LCQ) in solid-state mixtures using Raman mapping spectroscopy. B. Li, Y. Casamayou-Boucau, A. Calvet, and A.G. Ryder. Analytical Methods, 9, 6293-6301, (2017). DOI: 10.1039/C7AY01778B
- Kernel principal component analysis residual diagnosis (KPCARD): an automated method to remove cosmic ray artefacts in Raman spectra. B. Li, A. Calvet, Y. Casamayou-Boucau, A.G. Ryder, Analytica Chimica Acta, 913, 111-120, (2016). DOI: 10.1016/j.aca.2016.01.042.
- Low-content quantification in powders using Raman spectroscopy: a facile chemometric approach to sub 0.1% limits of detection., B. Li, A. Calvet, Y. Casamayou-Boucau, C. Morris, and A.G. Ryder, Analytical Chemistry, 87(6), 3419-3428, (2015). DOI: 10.1021/ac504776m
- Solid-state Transformations of Sulfathiazole Polymorphs: the Effects of Milling and Humidity, Y. Hu, A. Erxleben, B. Hodnett, B. Li, P. McArdle, A. Rasmuson, and A. G. Ryder, Crystal Growth & Design, 13(8), 3404-3413, (2013). DOI: 10.1021/cg4002779
- Quantitative Polymorph Contaminant Analysis in Tablets using Raman and Near Infra-red Spectroscopies. M. C. Hennigan and A. G. Ryder. Journal of Pharmaceutical and Biomedical Analysis, 72, 163-171, (2013). DOI: http://dx.doi.org/10.1016/j.jpba.2012.10.002 Download: Author version, Supplemental info.
- A comparative study of the use of powder X-ray diffraction, Raman and NIR spectroscopy for quantification of binary polymorphic mixtures of Piracetam, D.M. Croker, M.C. Hennigan, A. Maher, Y. Hu, A.G. Ryder, B.K. Hodnett. Journal of Pharmaceutical and Biomedical Analysis, 63, 80-86, (2012). DOI: 10.1016/j.jpba.2012.01.013
- Quantitative Analysis of Sulfathiazole Polymorphs in Ternary Mixtures by Attenuated Total Reflectance Infrared, Near-infrared and Raman Spectroscopy, Y. Hu, A. Erxleben, A.G. Ryder, and P. McArdle, Journal of Pharmaceutical and Biomedical Analysis, 53(3), 412-420, (2010). DOI: 10.1016/j.jpba.2010.05.002
- Determination of the polymorphic forms of bicifadine hydrochloride by DSC-TG, XRPD, ATR-IR, and ATR-NIR. P. McArdle, K. Gilligan, D. Cunningham, and A. Ryder, Applied Spectroscopy, 59(11), 1365-1371, (2005). DOI: 10.1366/000370205774783322
Presentations & posters:
- Invited: Raman Spectroscopy for particle sizing: more than just a compositional analysis tool. Nanotechnology Crossing Borders 2023, Hasselt Belgium. Oct. 24, 2023.
- Invited:Using Raman Spectroscopy to measure particle size of APIs in concentrated nanosuspensions. LONG-ACTING INJECTABLES CONFERENCE: Advances in manufacturing and monitoring suspension-based LAIs. Antwerp, Belgium, 8-9 February, 2023.
- Invited: Low Content and Multiple Analyte Quantification in the Solid-State by Raman Spectroscopy: An Alternative to HPLC? A. Ryder,* B. Li, A. Calvet, Y. Casamayou-Boucau, C. Morris, SciX2016, Hyatt Regency Hotel, Minneapolis, Mn. USA, 18-23 Sept., 2016.
- Plenary: Low-Concentration Analyte Quantification in the Solid State using Raman Spectroscopy and Chemometrics. 11th Australasian Conference on Vibrational Spectroscopy ( ACOVS11), University of Sydney, 29th of Sept. – 2nd Oct. 2015.
- Keynote: Low content quantification in solid powder matrix using Raman spectroscopy and chemometric methods. XV Chemometrics in Analytical Chemistry, Changsha, China, 22-26 June, 2015.
- Kernel Principal Component Analysis Residual Diagnosis Method For Removing Cosmic Ray Spikes From Raman Mapping Spectroscopic Data. B. Li,* and A.G. Ryder, XV Chemometrics in Analytical Chemistry, Changsha, China, 22-26 June, 2015.
- Raman and Near Infra-red Spectroscopic Analysis of Model Pharmaceutical Tablet Systems containing low levels of Polymorphic Contaminant. M.C Hennigan,* P. Matousek, and A.G Ryder. 38th FACSS, Reno, NV, 2-7 Oct., 2011.
- Spectroscopic Analysis of a Model Pharmaceutical Tablet System. M.C. Hennigan* and A.G. Ryder, RSC Analytical Research Forum 2011, University of Manchester, UK, 25 - 27 Jul., 2011.
- Determining the efficacy of rapid spectroscopic techniques for in-situ characterisation of polymorph contamination. M.C Hennigan,* Y. Hu, and A. G Ryder, 37th FACSS, Raleigh, NC, 17-21 Oct., 2010.
















