-
Courses

Courses
Choosing a course is one of the most important decisions you'll ever make! View our courses and see what our students and lecturers have to say about the courses you are interested in at the links below.
-
University Life

University Life
Each year more than 4,000 choose University of Galway as their University of choice. Find out what life at University of Galway is all about here.
-
About University of Galway

About University of Galway
Since 1845, University of Galway has been sharing the highest quality teaching and research with Ireland and the world. Find out what makes our University so special – from our distinguished history to the latest news and campus developments.
-
Colleges & Schools

Colleges & Schools
University of Galway has earned international recognition as a research-led university with a commitment to top quality teaching across a range of key areas of expertise.
-
Research & Innovation

Research & Innovation
University of Galway’s vibrant research community take on some of the most pressing challenges of our times.
-
Business & Industry

Guiding Breakthrough Research at University of Galway
We explore and facilitate commercial opportunities for the research community at University of Galway, as well as facilitating industry partnership.
-
Alumni & Friends

Alumni & Friends
There are 128,000 University of Galway alumni worldwide. Stay connected to your alumni community! Join our social networks and update your details online.
-
Community Engagement

Community Engagement
At University of Galway, we believe that the best learning takes place when you apply what you learn in a real world context. That's why many of our courses include work placements or community projects.
Protein-Surfaces
Probing Protein dynamics on surfaces:
 |
|
This research was initially funded (2003-08) by Science Foundation Ireland as part of a Principal Investigator award (02/IN.1/M231) and in part continued under the National Biophotonics Imaging Platform up to 2011 with Dr. Denisio Togashi being the lead researcher.
In 2012 an IRCSET funded PhD student (P. Zarski) continued with this research steam, developing Total Internal Reflection Microscopy (TIRFM) based methods for looking at protein-surface interactions.
In 2015 Camila van Zanten joined (funded by CAPES) and she is used advanced microscopy (FCS) to look at protein-nanoparticle interactions.
Protein-Surface Interactions:
When a complex biological system (a body, a tissue type, living cell, etc.) interacts with materials (e.g. engineered materials such as implant devices) it does so chemically and that process can be difficult to observe on a molecular level. The key interaction is that between the free proteins and the surface, and it is of major concern in a wide spectrum of fields such as biomaterials, nanotechnology, medicine, and biotechnological manufacturing processes. There is a need not only to measure the amount and rate of protein adsorption, but also the changes in protein structure. Whether protein adsorption is desirable or not, many fundamental features of protein-surface interactions still remain unknown, in particular information about protein structural changes during the adsorption process in complex environments.
This prompts questions about protein folding/unfolding, denaturation, orientation, stability, conformation and activity when coupled to surfaces. In many cases, these unknowns result from the fact that the tools are not readily available. Exploiting this gap in the underlying principles is required in order to control or engineer the protein-surface interaction. In this aspect, original ultra-sensitive approaches of detection, observation, and quantitative analysis must be developed and applied to provide meaningful, quantitative, and structural information. Furthermore, these approaches and models must be robust enough to allow for the heterogeneous nature of proteins and surfaces, and also take into special consideration the real-time kinetics and thermodynamics of conformational changes that occur as a protein encounters a surface. Understanding the underlying, fundamental processes that govern the interaction of biological systems with surfaces is key to the development of biocompatible medical devices which lead to successful implantable devices. This in turn translates to improved healthcare, minimally invasive surgery, healthier and prolonged lives.
In the NBL we have been investigating the use of fluorescence based methods for characterising and quantifying protein-surface interactions.
Research:
Our research is focused on developing quantitative fluorescence based methods to measure and analyze the interaction of proteins with different surfaces in real time. Some of the projects which we are undertaking (or have completed) include:
- Protein-Nanoparticle Interactions: Developing fluorescence based methods to study the interaction of nano-particles with proteins. This was part of a CAPES and a Hardiman funded PhD scholarship awards.
- Protein-Surface Interactions: Quantifying the amount of protein adsorbing on surfaces & understanding structural changes using advanced fluorescence microscopy (Total Internal Reflection Fluorescence).
- Protein-Protein interactions in living cells: As part of the Systems Biology Ireland Initiative we collaborated with Dr. H.-P. Nasheuer to measure protein-protein interactions in live cells using Fluorescence Correlation Spectroscopy (FCS). This is now concluded.
Current researchers:
Przemyslaw Zarski (PhD student, 2012-18): protein-surface interactions measured using TIRF microscopy.
Camila Van Zanten (PhD student, 2015-20): protein-nanoparticle interactions measured using FCS microscopy
Past senior researchers:
Dr. Denisio Togashi (2004-2011): protein-surface interactions.
Domhnall O'Shaughnessy (PhD student, protein-nanoparticle interactions).
Other Contributing researchers & students:
2009: Loretta Breslin and Neil Murphy (MSc project students), Edel Houghton (UREKA summer student)
2008: Amandine Calvet (project student), Valerie Murphy (4Y student).
2007: Muireann O'Loughlin (4Y student), Noemie Marguerite (French Undergraduate), Emmanuelle Bays (Swiss, IAESTE trainee.)
2006: Deirdre McMahon (MSc project student).
2005: Margaret Collins (MSc project student).
Publications & Conference Presentations:
-
Effects of viscosity and refractive index on the emission and diffusion properties of Alexa Fluor 405 using fluorescence correlation and lifetime spectroscopies., C. van Zanten, D. Melnikau, and A.G. Ryder. Journal of Fluorescence, 31(3), 835-845, (2021). DOI: 10.1007/s10895-021-02719-y
-
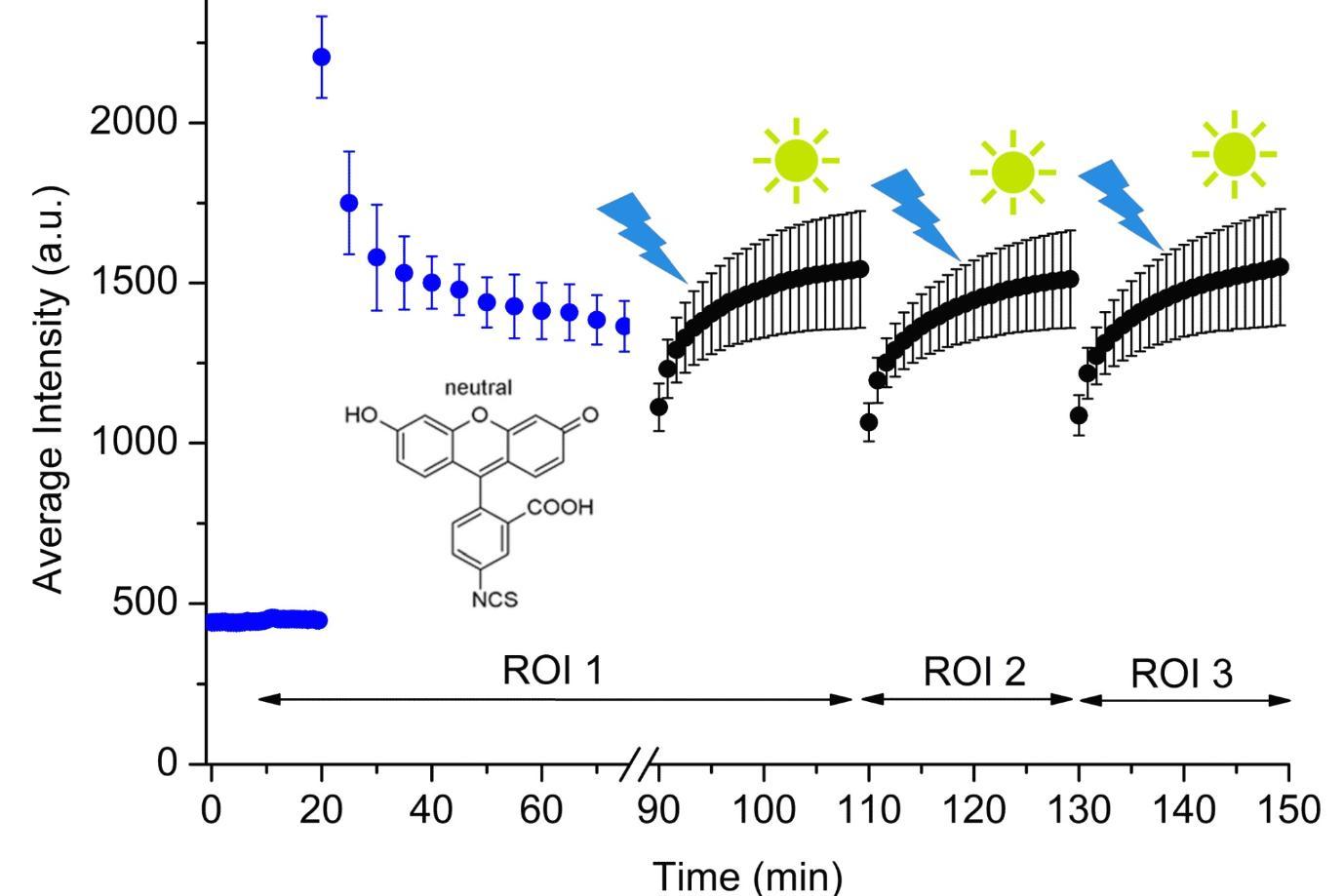
Super Stable Fluorescein Isothiocyanate Isomer I Monolayer for Total Internal Reflection Fluorescence Microscopy. P. Zarski and A.G. Ryder. Langmuir, 34(37), 10913-10923, (2018). DOI: 10.1021/acs.langmuir.8b02509
- Cell cycle-dependent mobility of Cdc45 in living cells determined by fluorescence correlation spectroscopy. R. Broderick, S. Ramadurai, K. Toth, D. Togashi, A. G. Ryder, J. Langwoski, and H.P. Nasheuer. PloS One, 7(4): e35537, (2012). DOI: 10.1371/journal.pone.0035537.
- Assessing protein-surface interactions with a series of multi-labeled BSA using Fluorescence Lifetime Microscopy and Förster Energy Resonance Transfer. D.M. Togashi and A.G. Ryder, Biophysical Chemistry, 152, 55-64, (2010). DOI: 10.1016/j.bpc.2010.07.006
- Monitoring Local unfolding of Bovine Serum Albumin during denaturation using steady-state and time-resolved fluorescence spectroscopy. D.M. Togashi, A.G. Ryder, and D. O'Shaughnessy, Journal of Fluorescence, 20(2), 441-452, (2010). DOI: 10.1007/s10895-009-0566-8
- Quantifying Adsorbed Protein on Surfaces using Confocal Fluorescence Microscopy. D.M. Togashi, A. G. Ryder, and G. Heiss, Colloids and Surfaces B: Biointerfaces. 72(2), 219-229, (2009).
DOI: 10.1016/j.colsurfb.2009.04.007 - Investigating trypthopan quenching of fluorescein fluorescence under protolytic equilibrium. D.M. Togashi, B. Szczupak, A.G. Ryder, A. Calvet, and M. OLoughlin, Journal of Physical Chemistry A, 113(12), 2757-2767, (2009). Online at ACS: http://pubs.acs.org/doi/full/10.1021/jp808121y .
- Trigger factor from the psychrophilic bacterium P. Frigidicola is a monomeric chaperone. S. Robin, D.M. Togashi, A.G. Ryder, and J.G. Wall, Journal of Bacteriology, 191(4), 1162-1168, (2009).
Online here. DOI: 10.1128/JB.01137-08 - A fluorescence analysis of ANS bound to bovine serum albumin: binding properties revisited by using energy transfer. D.M. Togashi and A.G. Ryder. Journal of Fluorescence, 18(2), 519-526, (2008).
Online here. DOI: 10.1007/s10895-007-0294-x. - Fluorescence Lifetime Imaging study of a thin protein layer on solid surfaces. D.M. Togashi and A.G. Ryder. Experimental & Molecular Pathology, 82(2). 135-141, (2007).
DOI: 10.1016/j.yexmp.2007.01.005 - Mobility and distribution of replication protein A in living cells at single molecule level. C. Braet, H. Stephan, I. Dobbie, D. Togashi, A.G. Ryder, Z. Foldes-Papp, N. Lowndes, and H.P. Nasheuer. Experimental & Molecular Pathology, 82(2). 156-162, (2007). DOI: 10.1016/j.yexmp.2006.12.008
- Time-resolved fluorescence studies on bovine serum albumin denaturation process. D.M. Togashi and A.G. Ryder, Journal of Fluorescence, 16(2), 153-160, (2006).
DOI: 10.1007/s10895-005-0029-9
Some Presentations:
- Using Fluorescence Correlation Spectroscopy (FCS) to measure protein stabilization by PNIPAm nanoparticles under mechanical stress. C. van Zanten, D. Melnikau, and A.G. Ryder, JJoint 12th EBSA, 10th ICBP-IUPAP Biophysics Congress, Madrid, 20-24 July, 2019. [Poster]
- Investigating the interaction of Alexa Fluor 405 and Atto 390 with PNIPAm using fluorescence correlation spectroscopy (FCS). C. Van Zanten,* D. Melnikau, A.G. Ryder. 15th Conference on Methods and Applications of Fluorescence: Spectroscopy, Imaging and Probes, MAF15, Bruges, Belgium, 10-13 Sept. 2017.
- Fluorescence Correlation Spectroscopy (FCS) studies of PNIPAm and Atto 390 systems in aqueous solutions. C. van Zanten,* D. Melnikau, and A.G. Ryder, NUI Galway/UL Alliance Research Day, 19th April, 2017.
- Monitoring adsorption of fluorescein iso-thiocyanate isomer I (FITC) on modified glass surface by total internal reflection fluorescent (TIRF) microscopy, P.M. Zarski,* A.G. Ryder, 14th Conference on Methods and Applications of Fluorescence: Spectroscopy, Imaging and Probes, Würzburg, Germany, 13–16 Sept., 2015.
- Using Total Internal Reflection Fluorescence Microscopy (TIRFM) for studying protein-surface interactions, 13th Conference on Methods and Applications of Fluorescence: Spectroscopy, Imaging and Probes, Genoa, Italy, 8 - 11 Sept., 2013.
- Study of Protein Deposition on Co-Polymers with different wetabilites by Confocal Fluorescence Microscopy, L. Breslin, D.M. Togashi, and A.G. Ryder, 42nd IUPAC Congress, Glasgow, 2-7 Aug. 2009.
- Probing the Structural Changes of Serum Albumin Protein in Thin Adsorbed Layers on Hydrophilic/Hydrophobic Surfaces using a FLIM-FRET approach. D.M. Togashi and A.G. Ryder. International Conference on Trends in Bioanalytical Sciences and Biosensors ( ICTBSB-2009), Dublin, 26-27 January 2009.
- Investigating Intramolecular Fluorescence Quenching in Serum Albumin Protein. M. O’Loughlin, D.M. Togashi, A.G. Ryder Europtrode IX, Dublin, March 30 - April 2, 2008.
- Confocal Fluorescence lifetime imaging (FLIM): a tool for analysis of structure changes of protein adsorbed onto solid surfaces. D.M. Togashi and A.G. Ryder. 10th Conference on Methods and Applications of Fluorescence: Spectroscopy, Imaging and Probes, Salzburg , Austria , 9-12 Sept., 2007.
- Binding Properties Revisited: a fluorescence analysis of ANS bound to bovine serum albumin. D.M. Togashi and A.G. Ryder. 10th Conference on Methods and Applications of Fluorescence: Spectroscopy, Imaging and Probes, Salzburg , Austria , 9-12 Sept., 2007. Poster available here.
- Fluorescence study of Bovine Serum Albumin and Ti and Sn Oxide Nanoparticles Interactions. D.M. Togashi, A.G. Ryder, D. Mc Mahon, P. Dunne, and J. McManus. European Conference on Biomedical Optics, Munich, Germany, 17-22 June, 2007.
- Evaluating the Structure Changes of Albumin Adsorbed onto Solid Matrix with Different Wettabilities by Fluorescence Lifetime Imaging (FLIM). D. Togashi and A. Ryder, 7th International ELMI meeting, York, England, 17-20 April, 2007.
- FCS and Fluorescent proteins: measuring diffusion rates, concentrations and protein-protein interactions. I. Dobbie, H. Stephan, H.-P. Nasheuer, D. Togashi, A. Ryder, and N. Lowndes. 7th International ELMI meeting, York, England, 17-20 April, 2007.
- Application of fluorescence lifetime imaging on adsorption of bovine serum albumin on solid surfaces. D.M. Togashi and A.G. Ryder. Microscopical Society of Ireland's 30th Annual Symposium, NUI-Galway, 30 Aug.-1 Sept., 2006.
- Real-time measurement of adsorption of bovine serum albumin on silica glass by confocal fluorescence microscopy. D.M. Togashi and A.G. Ryder. 6th International ELMI meeting, Ofir , Portugal , 30 May-2 June, 2006.
Methods for Studying Protein Surface Interactions:
This will be updated in the coming months. FLIM. TIRF.
|
|
We also have a range of other research projects where the equipment could be used for protein-surface interaction measurements, a more detailed list of equipment is given on the Instrumentation Pages.
Information Links:
These are some of the sites that we regularly use. I hope to add more links and details in the near future. If there are problems with any of the links let me know.
Technical Journals & Societies:
Surface Research & courses:
Polymer-protein surfaces, Switzerland.
Proteins-Polymers-Interfaces Group, at the Department of Bioengineering at the University of Utah.
Information Resources:
Equipment Links:
This is a selection of useful web-sites from a range of manufacturers who produce the equipment we use.
Olympus TIRF.
Ocean Optics (for fiber coupled spectrometers)
Ibidi (for disposables and chambers)