-
Courses

Courses
Choosing a course is one of the most important decisions you'll ever make! View our courses and see what our students and lecturers have to say about the courses you are interested in at the links below.
-
University Life

University Life
Each year more than 4,000 choose University of Galway as their University of choice. Find out what life at University of Galway is all about here.
-
About University of Galway

About University of Galway
Since 1845, University of Galway has been sharing the highest quality teaching and research with Ireland and the world. Find out what makes our University so special – from our distinguished history to the latest news and campus developments.
-
Colleges & Schools

Colleges & Schools
University of Galway has earned international recognition as a research-led university with a commitment to top quality teaching across a range of key areas of expertise.
-
Research & Innovation

Research & Innovation
University of Galway’s vibrant research community take on some of the most pressing challenges of our times.
-
Business & Industry

Guiding Breakthrough Research at University of Galway
We explore and facilitate commercial opportunities for the research community at University of Galway, as well as facilitating industry partnership.
-
Alumni & Friends

Alumni & Friends
There are 128,000 University of Galway alumni worldwide. Stay connected to your alumni community! Join our social networks and update your details online.
-
Community Engagement

Community Engagement
At University of Galway, we believe that the best learning takes place when you apply what you learn in a real world context. That's why many of our courses include work placements or community projects.
ARMES
Anisotropy Resolved Multidimensional Emission Spectroscopy (ARMES) and polarized Excitation Emission Matrix (pEEM) spectroscopy.
 |
Research Goals
Polarized Excitation Emission Matrix (pEEM) spectroscopy and Anisotropy resolved multidimensional emission spectroscopy (ARMES) and provides valuable insights into multi-fluorophore systems like proteins that have complex overlapping emission bands. The method combines multidimensional fluorescence, anisotropy/polarization, and chemometrics to facilitate the differentiation of fluorophores with very similar emission properties. The primary goal of the pEEM/ARMES projects are to develop a new high-information content measurement method that can provide a useful measurement modality for biopharmaceutical manufacturing and protein research.
In the ARMES based methods we use multivariate data analysis techniques like Multivariate Curve Resolution (MCR) or Parallel Factor (Parafac) analysis to try and resolve the individual emitting components based on different anisotropies.
In pEEM spectroscopy we do not resolve the individual components in a mixture but rather seek to use the unique advantages of the measurement for a range of specific applications, mostly focused in the area of biopharmaceutical manufacturing.
Current Research:
The MDF measurements and analytical methods comprise of two distinct elements, each with distinct benefits and applications:
- pEEM is the basic polarized Excitation-Emission Matrix measurements that are either perpendicular (EEM⟘) or parallel polarizations (EEMǁ). These measurements and their analysis by multi-way PCA and PLS based chemometric methods will form the basis of much of the analytical methodologies being developed with application in industrial biopharmaceutical manufacturing.
- Anisotropy Resolved Multidimensional Emission Spectroscopy (ARMES) is the combination of pEEM measurements with factor based chemometrics (MCR, PARAFAC, Tucker3, etc.) to extract individual emitting components and their scores. ARMES is more focused on modeling structure induced changes in emission properties of proteins.
- Insulin: Measuring aggregation state using pEEM.
- IgG: Measuring stability and aggregation state.
- PEG-IgG/ADC analogues: Investigating the possibility of using ARMES based method for reaction monitoring and the assessment of product quality.
- FRET modeling: Understanding how FRET affects ARMES data and developing a quantitative methodology. We are using a HSA-ANS system as an initial model.
- Chemometrics: Investigating the efficacy of existing methods for the analysis of polarized EEM/TSFS data. Developing a suite of analysis methodologies and tools for the analysis of this 4- and 5-way data.
- Instrumentation/Hardware: Modifying standard fluorimeters to enable ARMES data to be acquired over the full intrinsic fluorescence emission space of proteins. Building a lifetime measurement system to enable the measurement of nanosecond fluorescence lifetimes across the full emission space.
- Calibration & Validation: Developing the tools and protocols to ensure that the ARMES data and measurements are always accurate and correct.

Hardware/Instrumentation:
There are several aspects to the hardware and instrumentation required for ARMES. First to make accurate ARMES measurements in the UV region where most intrinsic fluorescence of proteins occurs we need to modify the polarizers found in most common spectrometers. Second once we collect ARMES data we have to validate the anisotropy element of the measurement and to do this we have built an Excitation-Emission Fluorescence Lifetime Spectrometer (EEFLS):|
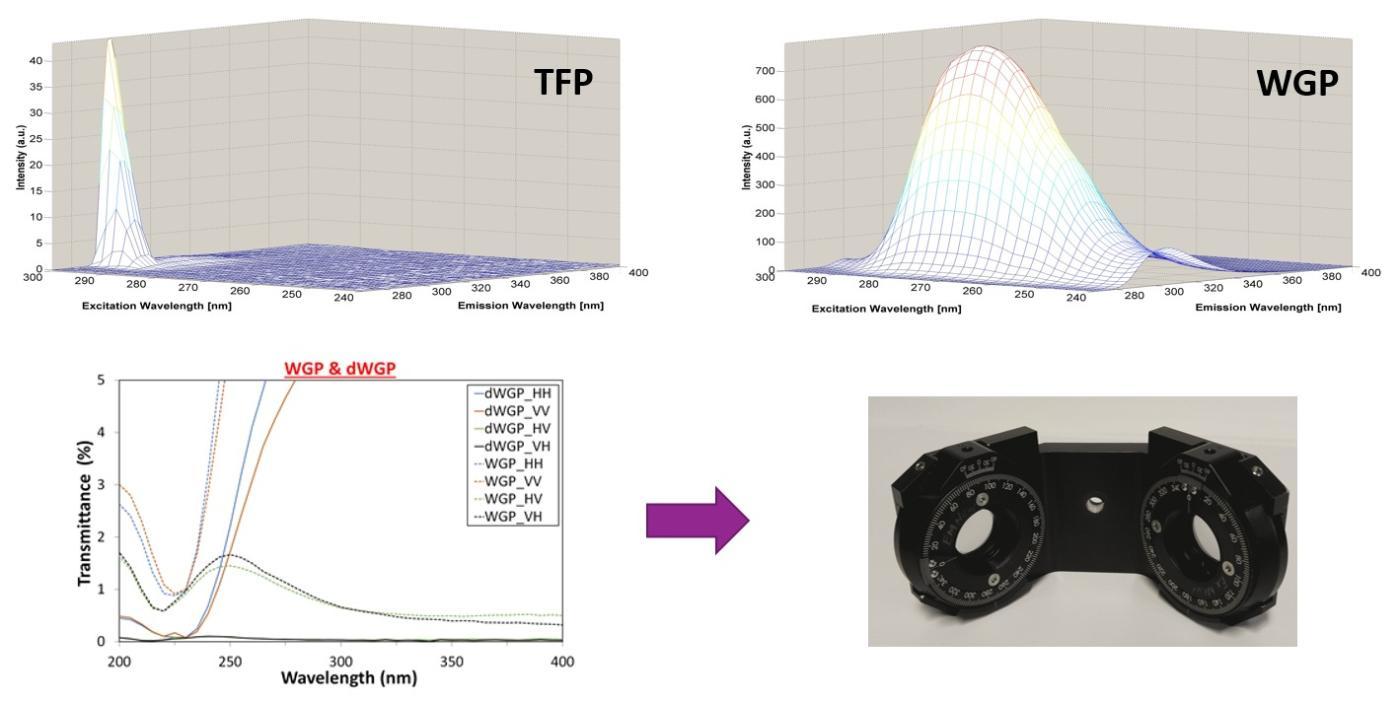
Polarizers: To fully access all of the intrinsic fluorescence emission space we need to use UV transparent polarizers. The figure on the right below shows the difference in transmission between the standard polymer based Thin Film Polarizers (TFP) and the dual Wire Grid Polarizers (dWGP) that we are using now. |
|
|
Full details can be found in our paper:
|
| Excitation-Emission Fluorescence Lifetime Spectrometer (EEFLS): This system was built to validate the anisotropy measurements across the complete emission space. It features a frequency doubled supercontinuum laser from Leukos. and a multi-anode (16 channel) detector from Becker & Hickl. The EEFLS has an Instrument Response Function (IRF) of ~650 ps in the UV which makes it suitable for accurate measurement of the nanosecond lifetimes of intrinsic protein fluorescence. |
| |
Full details of the EEFLS and its performance can be found in our recent paper:
|
Some Results:
We will upload more data and information as it comes available.
Proteins:
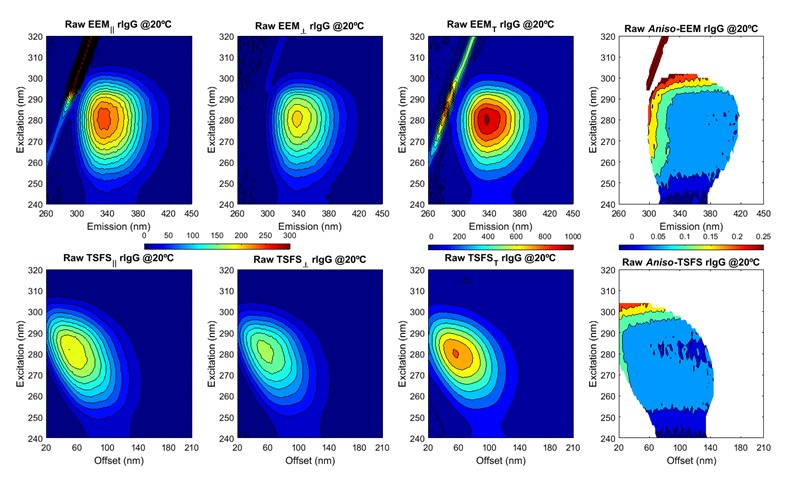 |
The plots above show ARMES data from an Immunoglobulin type protein. The data has been generated by two different measurement methods, Excitation-Emission Matrix (eem) and Total Synchronous Fluorescence Scan (TSFS). Each measurement method has its advantages and disadvantages in terms of the information content and interfering effects. This data has then to be mathematically analyzed using a variety of advanced multi-variate tools...and this is the chemometrics data analysis.
|
Chemometrics:
|
|
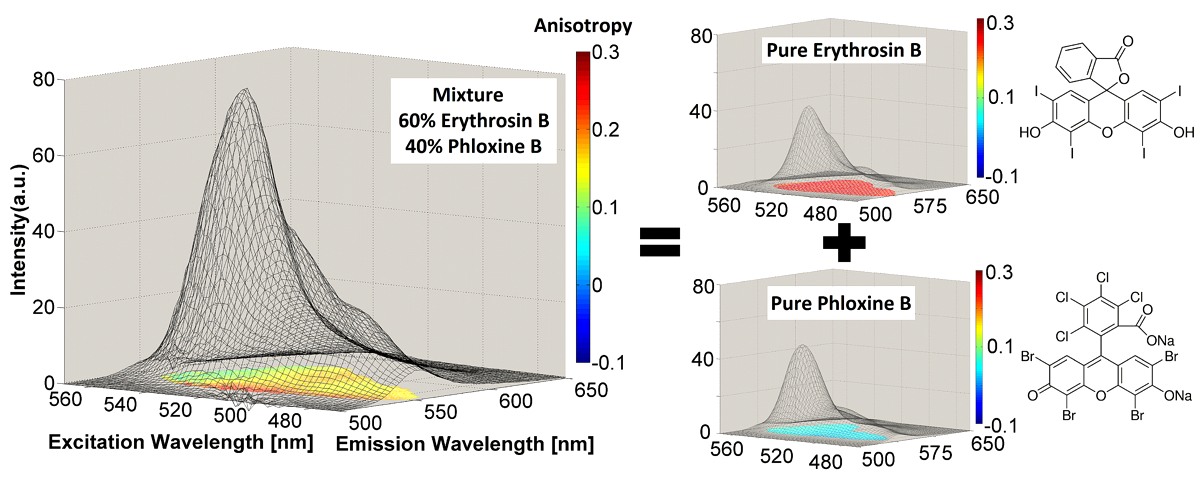
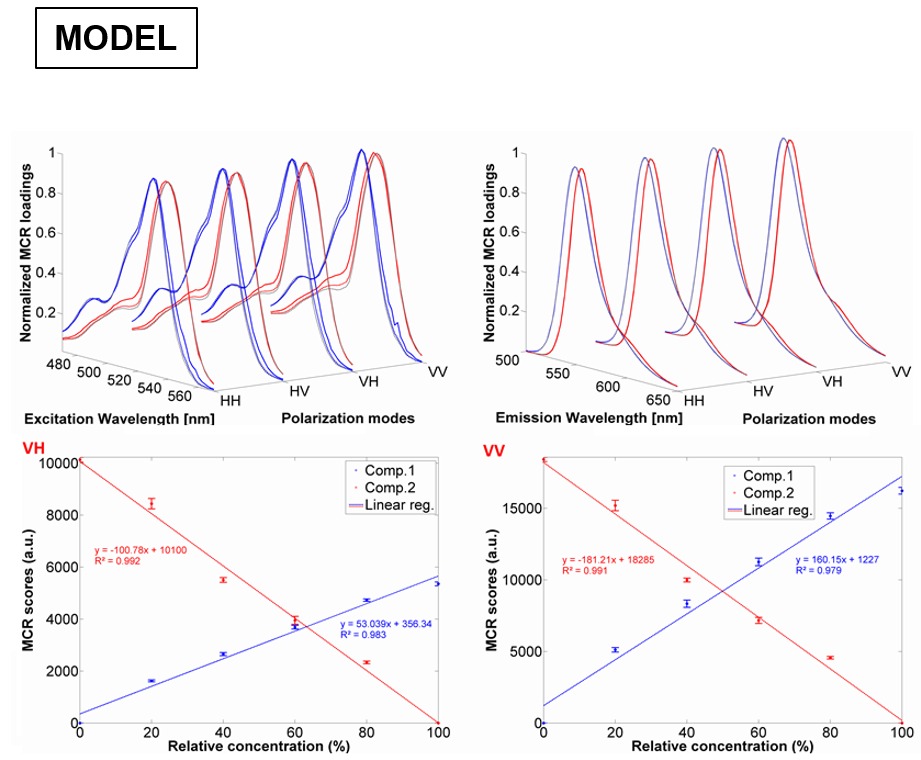
One of our goals is to be able to recover the correct excitation/emission spectrum of a fluorophore using chemometrics from mixtures of fluorophores with overlapping emission. The figure above shows an example of ARMES being applied to a small molecule model system. This system comprised of mixtures of Erythrosin B and Phloxine B. (Top row) Overlap between real excitation/emission spectra (full line-red/blue) and spectra loadings obtained from EB-PB-EEM-126 model (dash-grey line) and EB-PB-EEM-1256 model (dash-red/blue); (middle & bottom rows) MCR scores obtained for each pure component (EB-PB-EEM-1256 model) for the HH (c), HV (d), VH (e), and VV (f) dataset. Full details are in this paper:
|
Publications & Conference Presentations
More details, posters, and manuscripts will be uploaded as they become available. The works listed below are a combination of AA-BTM and PhD research papers.
Publications (updated 15/11/2022):
-
Analyzing protein conjugation reactions for Antibody-Drug Conjugate (ADC) synthesis using polarized Excitation Emission Matrix (pEEM) spectroscopy. A.-L. de Faria e Silva and A.G. Ryder. Biotechnology and Bioengineering, 119, 3432–3446, (2022). DOI: 10.1002/bit.28229. [Open access]
- Evaluating the interaction of human serum albumin (HSA) and 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) liposomes in different aqueous environments using Anisotropy Resolved Multi-Dimensional Emission Spectroscopy (ARMES). F. Gordon, Y. Casamayou-Boucau, A.G. Ryder.* Colloids and Surfaces B: Biointerfaces, 211, 112310, (2022). DOI: 10.1016/j.colsurfb.2021.112310. [Open access]
- Development of a rapid Polarized Total Synchronous Fluorescence Spectroscopy (pTSFS) method for protein quantification in a model bioreactor broth. B.O. Boateng, S. Elcoroaristizabal, and A.G. Ryder, Biotechnology and Bioengineering, 118(5), 1805-1817, (2021). DOI: 10.1002/bit.27694
- Modelling Förster Resonance Energy Transfer (FRET) using Anisotropy Resolved Multi-Dimensional Emission Spectroscopy (ARMES). F. Gordon, S. Elcoroaristizabal, and A.G. Ryder. Biochimica et Biophysica Acta - General Subjects, 1865(2), 129770, (2021): [Open Access] DOI: 10.1016/j.bbagen.2020.129770
- Quantitative analysis of weakly-bound insulin oligomers in solution using polarized multidimensional fluorescence spectroscopy. Y. Casamayou-Boucau and A.G. Ryder. Analytica Chimica Acta, 1138, 18-29, (2020). DOI: 10.1016/j.aca.2020.09.007. [Open Access]
- Characterization of Lysozyme PEGylation products using polarized Excitation‐Emission Matrix (pEEM) spectroscopy. A.-L. de Faria e Silva, S. Elcoroaristizabal, and A.G. Ryder. Biotechnology and Bioengineering, 117(10), 2969-2984, (2020). DOI: 10.1002/bit.27483
- Multi-Attribute Quality Screening of Immunoglobulin G using polarized Excitation Emission Matrix Spectroscopy. A.-L. de Faria e Silva, S. Elcoroaristizabal, and A.G. Ryder. Analytica Chimica Acta, 1101, 99-110, (2020). DOI: 10.1016/j.aca.2019.12.020 .
- Using Polarized Total Synchronous Fluorescence Spectroscopy (pTSFS) with PARAFAC analysis for Characterizing Intrinsic Protein Emission. M. Steiner-Browne, S. Elcoroaristizabal, and A.G. Ryder. Chemometrics and Intelligent Laboratory Systems, 194, 103871, (2019). DOI: 10.1016/j.chemolab.2019.103871
- Investigating Native State Fluorescence Emission of Immunoglobulin G using polarized Excitation Emission Matrix (pEEM) spectroscopy and PARAFAC. M. Steiner-Browne, S. Elcoroaristizabal, Y. Casamayou-Boucau, and A.G. Ryder. Chemometrics and Intelligent Laboratory Systems, 185, 1-11 (2019). DOI: 10.1016/j.chemolab.2018.12.007.
- An Excitation Emission Fluorescence Lifetime Spectrometer (EEFLS) system using a frequency doubled supercontinuum laser source. D. Melnikau, S. Elcoroaristizabal, and A.G. Ryder. Methods and Applications in Fluorescence, 6(4), 045007, (2018). DOI: 10.1088/2050-6120/aad9ae.
- Accurate anisotropy recovery from fluorophore mixtures using Multivariate Curve Resolution (MCR). Y. Casamayou-Boucau and A.G. Ryder. Analytica Chimica Acta. 1000, 132-143, (2018). DOI: 10.1016/j.aca.2017.11.031.
- Extended wavelength Anisotropy Resolved Multidimensional Emission Spectroscopy (ARMES) measurements: better filters, validation standards, and Rayleigh scatter removal methods. Y. Casamayou-Boucau and A.G. Ryder. Methods and Applications in Fluorescence, 5, 037001, (2017). DOI: 10.1088/2050-6120/aa7763
- Calibration, Standardization, and Quantitative Analysis of Multidimensional Fluorescence (MDF) Measurements on Complex Mixtures (IUPAC Technical Report). A.G. Ryder, C.A. Stedmon, N. Harrit, and R. Bro. Pure and Applied Chemistry , 89(12), 1849-1870, (2017). DOI: 10.1515/pac-2017-0610
- Anisotropy Resolved Multidimensional Emission Spectroscopy (ARMES): a new tool for protein analysis. R.C. Groza, B. Li, and A.G. Ryder. Analytica Chimica Acta, 886, 133-142, (2015). DOI: 10.1016/j.aca.2015.06.011.
- A Fluorescence Anisotropy Method for Protein Concentration Monitoring in Complex Cell Culture Media. R. C. Groza, A. Calvet, and A. G. Ryder. Analytica Chimica Acta, 821, 54-61, (2014). DOI: 10.1016/j.aca.2014.03.007
Relevant Invited Presentations (as of 08/04/2024):
- Polarized Excitation Emission Matrix (pEEM) spectroscopy for the rapid, non-destructive analysis of biological drug product and drug substances. 16th Annual PEGS Europe, Barcelona, Spain, 5-7 Nov., 2024.
- Using Polarized Excitation Emission Matrix (pEEM) for monitoring protein conjugation reactions. SCIX2023, Sparks Nevada, USA, Oct. 8-13, 2023.
- Polarized Excitation Emission Matrix (pEEM) spectroscopy: another dimension for protein analysis. SCIX2023, Sparks Nevada, USA, Oct. 8-13, 2023.
- The application of multi-dimensional fluorescence spectroscopy, anisotropy, and chemometrics as a process-wide, analytical technology platform for Biopharmaceuticals. EuChemS Chemistry Congress. Lisbon, Portugal, 30 Aug. – 03 Sept., 2022.
- Polarized Excitation Emission Matrix (pEEM) spectroscopy for therapeutic protein analysis: making anisotropy work in a multi-dimensional way. Pittcon 2021.
- Using Intrinsic Fluorescence Emission for Analysis of Cell Culture Media and Protein-Based Samples, Bioprocessing Summit Europe, Virtual Conference, 21-23, July, 2020. [Analytical Characterisation stream]
- Anisotropy Resolved Multi-Dimensional Emission Spectroscopy (ARMES): A Multivariate Approach to Intrinsic Protein Emission Analysis, 11th Annual PEGS Europe, Lisbon, Portugal, 18-22 Nov., 2019.
- Bioprocess monitoring and protein quality assessment using polarized intrinsic fluorescence spectroscopy in multi-dimensional modes: a new measurement methodology. Analyzing / Monitoring Processes to Ensure Quality at The Bioprocessing Summit, Seaport World Trade Center, Boston, Mass., USA, 14-15 Aug., 2019.
- Anisotropy Resolved Multi-dimensional Emission Spectroscopy (ARMES) for the analysis of proteins in solution: a new analytical paradigm, A.G. Ryder, BioProduction Congress, Dublin, 9-10 Oct. 2018 (invited).
- Using multi-dimensional fluorescence spectroscopy measurements in Biopharmaceutical Manufacturing: the need for better multidimensional standards,” A.G. Ryder, 15th International Symposium on Biological and Environmental Reference Materials (BERM 15), Berlin, Germany, 24-26 Sept., 2018 (invited).
- Complexity, the Bugbear Preventing Synergy Between Real Time Analytics in Small vs. Large Molecules. A.G. Ryder. IFPAC-2018, Bethesda, MD, USA, 11-14 Feb. 2018 (invited).
- Developing multi-dimensional fluorescence spectroscopies as a process-wide. platform analytical technology for Biopharmaceutical analysis. A.G. Ryder, EuPAT8, Cork, Ireland, 3-4 Oct. 2016 (invited).
- The use of multi-dimensional fluorescence spectroscopy for the quantitative analysis of liquid media: from hydrolysates to protein solutions. Eastern Analytical Symposium, Somerset, NJ, USA, 16-18 Nov., 2015 (invited).
ARMES/pEEM Conference Poster & Oral Presentations (as of 08/04/2024):
- "Measuring Protein-polymer nanoparticle interactions using polarized Excitation Emission Matrix (pEEM) spectroscopy." Matheus de Castro* & Alan G. Ryder. SCIX2023, Sparks Nevada, USA, Oct. 8-13, 2023.
- "Use of polarised Total Synchronous Fluorescence Spectroscopy (pTSFS) for quantitative bioprocess monitoring of IgG antibody in a complex model bioreactor broth." Bernard Boateng* and Alan Ryder. Analytical Research Forum, 15 June, 2021. Online presentation.
- "Characterizing PEGylation Products Using Polarized Excitation Emission Matrix (pEEM) Spectroscopy." A.-L. de Faria e Silva,* S. Elcoroaristizabal, A.G. Ryder. Bioprocessing Summit Europe, Virtual Conference, 21-23, July, 2020. [P09, Poster]
- "Multi-Attribute Quality Screening of Immunoglobulin G Using Polarized Excitation Emission Matrix Spectroscopy." A.-L. de Faria e Silva,* S. Elcoroaristizabal, A.G. Ryder. Bioprocessing Summit Europe, Virtual Conference, 21-23, July, 2020. [P21, Poster]
- "Anisotropy Resolved Multidimensional Emission Spectroscopy (ARMES) and Chemometric Modelling to Study Förster Resonance Energy Transfer (FRET) Processes." F. Gordon*, S. Elcoroaristizabal, A.G. Ryder. 64th Annual Meeting of the Biophysical Society, San Diego, USA, Feb. 2020.
- "Investigating Native State Fluorescence Emission of Immunoglobulin G (IgG) using polarized Excitation Emission Matrix (pEEM) spectroscopy and PARAFAC." M. Steiner-Browne,* S. Elcoroaristizabal, Y. Casamayou-Boucau, and A.G. Ryder. Joint 12th EBSA, 10th ICBP-IUPAP Biophysics Congress, Madrid, 20-24 July, 2019.
- "Using Anisotropy Resolved Multidimensional Emission Spectroscopy (ARMES) and chemometric modelling to study Förster Resonance Energy Transfer (FRET) processes." F. Gordon, S. Elcoroaristizabal and A.G. Ryder, Joint 12th EBSA, 10th ICBP-IUPAP Biophysics Congress, Madrid, 20-24 July, 2019.
- "Anisotropy Resolved Multidimensional Emission Spectroscopy (ARMES) as a Tool for Characterization of Mixed Protein Solutions." S. Elcoroaristizabal and A.G. Ryder, Joint 12th EBSA, 10th ICBP-IUPAP Biophysics Congress, Madrid, 20-24 July, 2019.
- "Protein Quantification in a model bioreactor broth using polarized Total Synchronous Fluorescence Spectroscopy (pTSFS)." B. Boateng,* S. Elcoroaristizabal, A.G. Ryder. IFPAC-2019, Bethesda, MD, USA, 3-6 Mar. 2019 (oral pres.).
- "Assessing IgG PEGylation reactions and products using polarized Excitation Emission Matrix (pEEM) spectroscopy." A.-L. de Faria e Silva,* S. Elcoroaristizabal, A.G. Ryder. IFPAC-2019, Bethesda, MD, USA, 3-6 Mar. 2019 (oral pres.).
- "Assessing IgG quality using polarized Excitation Emission Matrix (pEEM) spectroscopy." A.-L. de Faria e Silva,* S. Elcoroaristizabal, A.G. Ryder. IFPAC-2019, Bethesda, MD, USA, 3-6 Mar. 2019. [2nd prize in poster competition].
- "Effect of signal to noise ratio on polarized Total Synchronous Fluorescence Spectroscopy (pTSFS) measurements used for bioprocess analysis." B. Boateng,* S. Elcoroaristizabal, A.G. Ryder. IFPAC-2019, Bethesda, MD, USA, 3-6 Mar. 2019. [Poster]
- "Assessing freeze-thaw effects in IgG using Anisotropy Resolved Multi-Dimensional Emission Spectroscopy (ARMES)." M. Steiner-Browne,* S. Elcoroaristizabal, Y. Casamayou-Boucau, and A.G. Ryder. BioPharma Ambition 2018 , Dublin Castle, 21-11 Feb. 2018. [Winning poster].
- "A new rapid analytical methodology for soluble insulin aggregate analysis." Y. Casamayou-Boucau* and A.G. Ryder. BioPharma Ambition 2018, Dublin Castle, 21-11 Feb. 2018.
- "Characterizing PEGylation reactions using multidimensional fluorescence (MDF) spectroscopy." A.L. de Faria*, S. Elcoroaristizabal, Y. Casamayou-Boucau, and A.G. Ryder. BioPharma Ambition 2018, Dublin Castle, 21-11 Feb. 2018.
- “Monitoring Structural Stability and Aggregation of Immunoglobulin G (IgG) Under Thermal Stress using Anisotropy Resolved Multidimensional Emission Spectroscopy (ARMES).” M. Steiner,* S. Elcoroaristizabal, Y. Casamayou-Boucau, and A.G. Ryder. 15th Conference on Methods and Applications of Fluorescence: Spectroscopy, Imaging and Probes, MAF15, Bruges, Belgium, 10-13 Sept. 2017.
- “Anisotropy resolved multidimensional emission spectroscopy (ARMES) for discrimination of mixed serum albumin solutions.” S. Elcoroaristizabal* and A.G. Ryder. 15th Conference on Methods and Applications of Fluorescence: Spectroscopy, Imaging and Probes, MAF15, Bruges, Belgium, 10-13 Sept. 2017.
- “Characterizing IgG conjugation products using Anisotropy Resolved Multi-Dimensional Spectroscopy (ARMES).” A.L. de Faria,* S. Elcoroaristizabal, Y. Casamayou-Boucau, and A.G. Ryder. 1 15th Conference on Methods and Applications of Fluorescence: Spectroscopy, Imaging and Probes, MAF15, Bruges, Belgium, 10-13 Sept. 2017.
- Anisotropy Resolved Multi-dimensional Emission Spectroscopy (ARMES): A new tool for protein structure analysis, R.C. Groza, B. Li, and A.G. Ryder. 14th Conference on Methods and Applications of Fluorescence: Spectroscopy, Imaging and Probes, Würzburg, Germany, 13–16 Sept., 2015.
- Analysis of cell culture media by multi-dimensional fluorescence anisotropy. R. C. Groza and A. G. Ryder, 13th Conference on Methods and Applications of Fluorescence: Spectroscopy, Imaging and Probes, Genoa, Italy, 8 – 11 Sept., 2013.
Collaborations & funding
We are happy to work with industrial partners to help apply these methods to protein analysis challenges in biopharma.
For Bio-pharmaceutical companies based in Ireland there are a variety of support mechanisms:
- Enterprise Ireland offer a range of supports to leverage additional support for Industry-Academia partnerships.
- The Irish Research Council also offer the possibility of jointly funded PhD scholarships and postdoctoral posts under the Enterprise Partnership Scheme.
- SFI also have a Technology and Innovation Development Award (TIDA) which can be used to support short 1 year, research projects.
 Tra Na Rossan, na Dunaibh.
Tra Na Rossan, na Dunaibh.
Contact:
Nanoscale Biophotonics Laboratory, Room 213,
University of Galway, University Road, Galway, H91 CF50, Ireland.
Tel: +353 (0)91 492943 or ext. 2943 (internal) E-mail: alan.ryder
 nuigalway.ie
nuigalway.ie