-
Courses

Courses
Choosing a course is one of the most important decisions you'll ever make! View our courses and see what our students and lecturers have to say about the courses you are interested in at the links below.
-
University Life

University Life
Each year more than 4,000 choose University of Galway as their University of choice. Find out what life at University of Galway is all about here.
-
About University of Galway

About University of Galway
Since 1845, University of Galway has been sharing the highest quality teaching and research with Ireland and the world. Find out what makes our University so special – from our distinguished history to the latest news and campus developments.
-
Colleges & Schools

Colleges & Schools
University of Galway has earned international recognition as a research-led university with a commitment to top quality teaching across a range of key areas of expertise.
-
Research & Innovation

Research & Innovation
University of Galway’s vibrant research community take on some of the most pressing challenges of our times.
-
Business & Industry

Guiding Breakthrough Research at University of Galway
We explore and facilitate commercial opportunities for the research community at University of Galway, as well as facilitating industry partnership.
-
Alumni & Friends

Alumni & Friends
There are 128,000 University of Galway alumni worldwide. Stay connected to your alumni community! Join our social networks and update your details online.
-
Community Engagement

Community Engagement
At University of Galway, we believe that the best learning takes place when you apply what you learn in a real world context. That's why many of our courses include work placements or community projects.
Past Projects
Fluorescence Lifetime based pH sensing
 |
Objectives:
This project aimed to develop high accuracy optical pH sensors suitable for biological and clinical applications. We used fluorescence lifetime methods because they offer a convenient route to the development of minimally invasive monitoring methods. The fluorescence lifetime is the average time that a molecule spends in the excited state before emitting a photon of light and returning to the ground state; most biological molecules have lifetimes in the picosecond (10 -12 sec) to microsecond (10 -6 sec) range. Fluorescence lifetime measurements are independent of signal level and have been used to obtain pH measurements from cellular material and to measure oxygen content through skin [1,2]. This is a significant advantage compared to optical sensors that rely on fluorescence intensity and/or wavelength measurements, which can be adversely affected by light scattering/absorption effects due to tissue inhomogeneity. This work ws done by Dr. Sarah Power as her PhD research project from 1999 to 2003.
Our pH sensors were based on relatively short-lived species with nanosecond lifetimes, and the primary fluorophore was acridine. This molecule exhibits a large difference in lifetime between the protonated and base forms making it a candidate for pH sensing. There are however a number of difficulties such as excited state protonation and halide quenching to be overcome before acridine can be used as a pH sensor [ 3,4]. We also examined resorufin as a possible lifetime based pH sensor using 460 nm LED excitation [ 5].
Acridine in 0.1 M phosphate buffer:
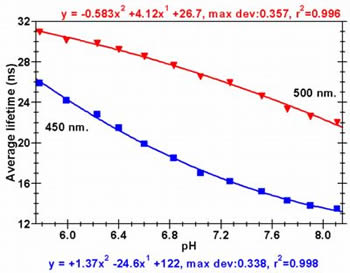
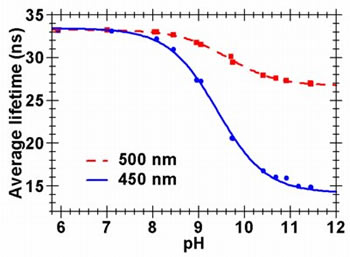 Acridine is a pH sensitive dye that exhibits a large difference in fluorescence lifetime, between the neutral and protonated states. The plot on the left shows the difference in the fluorescence decay curves (380 nm excitation) for acridine in 0.1 M phosphate buffer at two different pH values. Acridine is a pH sensitive dye that exhibits a large difference in fluorescence lifetime, between the neutral and protonated states. The plot on the left shows the difference in the fluorescence decay curves (380 nm excitation) for acridine in 0.1 M phosphate buffer at two different pH values. It is clear that each decay curve comprises of two emitting species, and that the proportion of each changes with pH. The protonated species has a lifetime of ~31.6 ns in 1M HNO3 while the neutral base form has a lifetime of 6.6 ns in 1 M NaOH. |
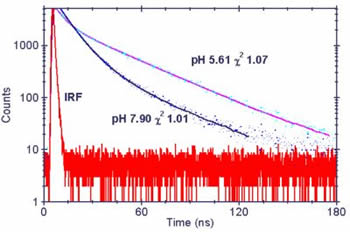 If we plot the change in the measured average lifetime versus pH at two different emission wavelengths we get the plots on the right. The relationship between pH and average lifetime fits to a simple polynomial over the physiologically important pH 5.8 - 8.1 range. If we plot the change in the measured average lifetime versus pH at two different emission wavelengths we get the plots on the right. The relationship between pH and average lifetime fits to a simple polynomial over the physiologically important pH 5.8 - 8.1 range. The average lifetime is longer at longer emission wavelengths because the protonated form of acridine emits at longer wavelength than the neutral form. |
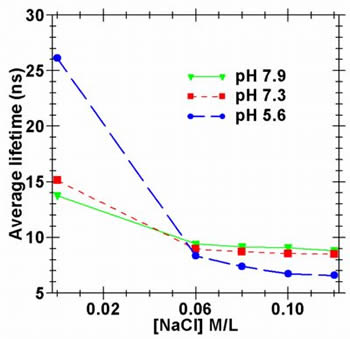 Unfortunately acridine fluorescence can be quenched by the presence of species such as halide ions. The plots opposite show the difference in average lifetime (measured at 450 nm) for acridine (5 x 10-3 M) in solution when NaCl is added. The dramatic drop in the average lifetime is due to the dynamic quenching of the protonated species. Unfortunately acridine fluorescence can be quenched by the presence of species such as halide ions. The plots opposite show the difference in average lifetime (measured at 450 nm) for acridine (5 x 10-3 M) in solution when NaCl is added. The dramatic drop in the average lifetime is due to the dynamic quenching of the protonated species. This along with the fact that acridine undergoes an excited state protonation makes acridine unsuitable as a pH sensor except under very well controlled circumstances. Our next step was therefore to find some way of negating the halide quenching and excited state effects, and to do this we deployed a protective support material. |
Protected Acridine:
|
The shift in ground state pKa is due to the unique environments that acridine experiences in the membrane. This large shift (~ 4 pH units) in the ground state pKa ensures that ground state protonation is not an issue any more. [ note the pKa values for solution and membrane acridine are dependant on the emission wavelength sampled] |
References:
1). Quantitative pH imaging in cells using confocal fluorescence lifetime imaging microscopy. S. Sanders, A. Draaijer, H.C. Gerritsen, P.M. Houpt, & Y.K. Levine, Anal. Biochem., 227, 302-308, (1995).
2). Sensing oxygen through skin using a red diode laser and fluorescence lifetimes. G. Rao et al., Biosensors & Bioelectronics, 10, p. 643-, (1995).
3). Time-domain measurement of fluorescence lifetime variation with pH. A.G. Ryder, S. Power, T.J. Glynn, & J.J. Morrison. Proc SPIE vol. 4259, pp. 102-109, (2001).
4). Evaluation of acridine in Nafion as a fluorescence lifetime based pH sensor. A.G. Ryder, S. Power, a& T.J. Glynn. Applied Spectroscopy, 57(1), 73-79, 2003.
5). Fluorescence lifetime based pH sensing using Resorufin. A.G. Ryder, S. Power, & T.J. Glynn. Proc SPIE vol. 4876, 827-835, (2003). .
STARs Science Teacher Assistant Researcher
 My name is Yvonne Higgins and I work as a science teacher in East Glendalough secondary school in Co. Wicklow. This summer, I am working in the NCBES in NUI Galway for a period of eight weeks as part of the STARs project. It was at an Irish Science Teachers Association ( ISTA) meeting that I first heard about the STARs programme.
My name is Yvonne Higgins and I work as a science teacher in East Glendalough secondary school in Co. Wicklow. This summer, I am working in the NCBES in NUI Galway for a period of eight weeks as part of the STARs project. It was at an Irish Science Teachers Association ( ISTA) meeting that I first heard about the STARs programme.
This programme, introduced this year by SFI, provides secondary school science teachers with an opportunity to work with research groups in third level institutions. I decided to take part in this programme as I recognised it as a wonderful opportunity to enhance my skills as a science teacher. Above all, it would allow me to introduce my students to the world of cutting-edge research right here in Ireland!
Thus, I set about finding a project that would encompass my two main areas of interest; chemistry and biology. Firstly, I accessed the STARs projects on the SFI website. Here, I came across a list of projects from various scientific disciplines, but it was the following one that caught my attention; Fluorescence Lifetime Based Sensing: Methods and Instrumentation. This project is located in the Nanoscale Biophotonics laboratory, under the direction of Dr. Alan G. Ryder. The next step was to contact Dr. Ryder, and a joint application was made to SFI, which thankfully, was accepted!
What are the benefits of such a scheme? Personally, I have found that:
- My laboratory skills have been improved - which is important to those teachers working in schools lacking laboratory technicians.
- Being involved in genuine research, as opposed to following text-book instructions, will be of benefit with assisting students undertaking Young Scientist projects.
- It enables me to keep up-to-date with the most recent developments in research and industry.
- The knowledge that I gain will help me to make my students aware of the potential job prospects for science graduates.
- The fact that many of the concepts that science students examine, for example; acids and bases, pH, absorption, and emission spectra and light are relevant to this project underlines the importance of learning such core principles.
- I can also inform my chemistry students that as part of the project, I used some of the laboratory skills that they have already learned, such as making up solutions.
- Taking part in such a project means that I have built greater contacts between my school and third level institutions. Such contacts are of importance to universities as it means that the work carried out in their institutions is disseminated among possible future undergraduates. In addition, the teachers involved further the work being carried out by the research group.
Finally, I have thoroughly enjoyed my work experience in the NCBES and would recommend any science teacher to take part in the STARs programme. In addition to the many advantages mentioned above, it has provided me with an opportunity to work with scientists and engineers of many different nationalities including Australian, French, Norwegian, and Polish. All I can say is that I look forward to the prospect of taking part in further projects!
O ne of the researchers I am working with is an IAESTE (International Association for the Exchange of Students for Technical Experience) exchange student from Australia, Joanne Gashumba. Joanne is an undergraduate student studying science and engineering at Monash University, Melbourne Australia. She is currently completing a 12 week internship in the same lab. working on this project " Development of Fluorescence lifetime based sensors". Joanne is carrying out some of the more Physics orientated tasks of this interdisciplinary project. ne of the researchers I am working with is an IAESTE (International Association for the Exchange of Students for Technical Experience) exchange student from Australia, Joanne Gashumba. Joanne is an undergraduate student studying science and engineering at Monash University, Melbourne Australia. She is currently completing a 12 week internship in the same lab. working on this project " Development of Fluorescence lifetime based sensors". Joanne is carrying out some of the more Physics orientated tasks of this interdisciplinary project. |
Research project
Aim: The overall goal of this research is to develop a real-time, minimally invasive pH sensor for monitoring foetal blood pH during labour. This involves the use of a fluorescent dye, acridine, which exhibits a large difference in fluorescent lifetime between the neutral and protonated states, making it an ideal candidate for pH sensing. The fluorescent lifetime of a molecule is the average time it spends in the excited state before returning to the ground state. Nafion, a perfluorinated ionomer polymer, is being used as a matrix for the dye.
Project work:
Before I commenced any practical work, I had to prepare all the necessary glassware. All glass-ware was washed with detergent and warm water, rinsed several times with water and then four times with deionised water. Finally, all glass-ware was rinsed with acetone and dried.
Solutions were prepared as follows:
10 -4 M acridine solution: dissolving 0.00448g of acridine in 250ml ethanol.
1M HNO 3: 64.286ml concentrated HNO 3 in 1L using deionised water.
1M NaOH: dissolving 40g NaOH in 1L deionised water.
200mM stock solution of Na 2HPO 4: dissolving 53.614g Na 2HPO 4.7H 2O in 1L deionised water.
200mM stock solution of NaH 2PO 4: dissolving 27,598g NaH 2PO 4.H 2O in 1L deionised water.
Buffered solutions:
For pH 5.8-8.0, buffered solutions were made up according to the following table:
|
pH |
200mM Na 2HPO 4(ml) |
200mM NaH 2PO 4 (ml) |
Deionised water (ml) |
|---|---|---|---|
|
5.8 |
4.0 |
46.0 |
40 |
|
6.0 |
6.15 |
43.85 |
40 |
|
6.2 |
9.25 |
40.75 |
40 |
|
6.4 |
13.25 |
36.75 |
40 |
|
6.6 |
18.75 |
31.25 |
40 |
|
6.8 |
24.5 |
25.5 |
40 |
|
7.0 |
30.5 |
19.5 |
40 |
|
7.2 |
36.0 |
14.0 |
40 |
|
7.4 |
40.5 |
9.5 |
40 |
|
7.6 |
43.5 |
6.5 |
40 |
|
7.8 |
45.75 |
4.25 |
40 |
|
8.0 |
47.35 |
2.65 |
40 |
At pH values above 8, a 100mM stock buffer solution was made up by dissolving 26.807g Na 2HPO 4.7H 2O in 800ml deionised water. This solution was divided into 80ml samples, these were then titrated to the following pH values; 8.5, 9.0, 9.5, 10.0, 10.5, 11.0, 11.5 and 12.0 using either 1M HNO 3 or 1M NaOH, and made up to 100ml with deionised water.
Purification of Nafion film:
Ten strips of nafion film were cut to fit 1mm quartz cuvettes. These were placed in concentrated HNO 3 and stirred at 60°C for 24hr. The film was then placed in 60, 40, and 20% nitric acid respectively and stirred for 1hr in each. The film was then washed thoroughly with deionised water. The nafion strips were converted to the sodium form by placing them in 0.1M NaOH solution with stirring for 24hr. Once again, the film was washed thoroughly with deionised water.
Incorporating acridine into nafion film:
The films were then washed three times with ethanol and left to stand for 5 minutes in each rinse. Acridine was then incorporated into the films by placing them in 20ml 1 x 10 -4 M acridine solution for 5 minutes. Following this, the films were rinsed three times with ethanol before finally being rinsed thoroughly with deionised water. The films were stored in deionised water until use.
Adjusting film to the desired pH:
Before recording absorption and emission spectra and fluorescence lifetimes, the film was placed in buffer solution of the required pH for 30 minutes, replacing the solution after 10, 20, and 25 minutes. The film was then placed in a 1mm quartz cuvette containing the same pH buffer. Following the recording of each spectrum, the film was removed and washed thoroughly with deionised water.
| UV-Visible Absorption and Fluorescence Emission spectroscopy: Absorption spectra were recorded using a UV-1601 uv-visible spectrophotometer (below left). The samples were scanned over the following range of wavelengths; 550 - 300nm. The wavelength of maximum absorption was noted in each case. Samples were then excited at this wavelength in order to record their emission spectra. Emission spectra were recorded on a Perkin-Elmer luminescence spectrophotometer (below right). The emission spectra were employed to determine the appropriate wavelengths at which to measure the fluorescence lifetimes.Picture: At work in the laboratory inserting the Nafion strips into cuvettes for spectroscopic measurements.
|
|
|
Fluorescence Lifetime Measurements The average fluorescent lifetime of a molecule is the average time the molecule spends in the excited state before returning to the ground state. These lifetimes are very short, from the picosecond (10 -12) to microsecond (10 -6) range. The fluorescence lifetimes of acridine in buffer and acridine in Nafion were found as a function of pH for different emission wavelengths. The FluoTime 200 Fluorescence Lifetime Spectrometer (right) was used to obtain lifetime measurements. Initially a 380nm LED excitation source was used for exciting the samples of acridine in buffer for the physiologically important pH range of 5.7 to 8. For obtaining the average lifetimes of acridine in Nafion, a 375nm laser source was used at an intensity of 50% - measurements were taken for the pH range of 8 - 12 rather than for the pH range of 6 - 8 as the greatest change in average lifetime for acridine in Nafion occurs during the former pH range. The instrument response function was measured using a diluted solution of silica and the decay curves for a number of different wavelengths were also recorded using this same software. The picture (left) shows Joanne using the FluoTime 200. |
Some results:
|
|
|
The plots to the left show the change in the average lifetime for the pH range of 5.7 to 8 which is of physiological importance. |
|
Acridine fluorescence can be quenched by certain species such as halide ions making it an unsuitable pH sensor. |








 We have incorporated acridine into a transparent, anion exclusion membrane in order to prevent halide quenching. Preliminary results are shown on the right.
We have incorporated acridine into a transparent, anion exclusion membrane in order to prevent halide quenching. Preliminary results are shown on the right. 


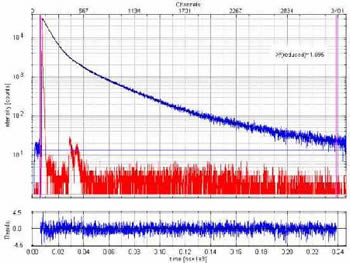 Acridine is a very pH sensitive dye. The figure to the right shows the fluorescence decay curve (in Violet excitation 375nm) of a solution of acridine in buffer and the Instrument Response Function of the FluoTime 200 in Red. The pH value of the solution was 6.54 and the emission wavelength was 440nm.
Acridine is a very pH sensitive dye. The figure to the right shows the fluorescence decay curve (in Violet excitation 375nm) of a solution of acridine in buffer and the Instrument Response Function of the FluoTime 200 in Red. The pH value of the solution was 6.54 and the emission wavelength was 440nm. 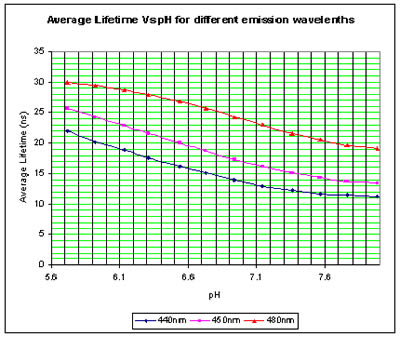 Lifetime Measurements - Acridine in Buffer
Lifetime Measurements - Acridine in Buffer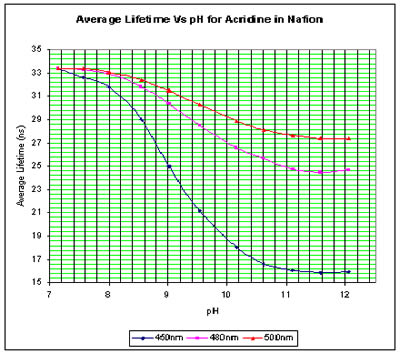 Lifetime Measurements - Acridine in Nafion
Lifetime Measurements - Acridine in Nafion